"V体育官网" Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation
- PMID: 10655519
- PMCID: PMC15590
- DOI: 10.1073/pnas.97.3.1263 (V体育官网入口)
Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation
Abstract (VSports手机版)
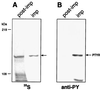
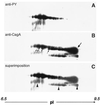
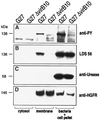
Helicobacter pylori strains associated with severe tissue damage and inflammation possess a unique genetic locus, cag, containing 31 genes originating from a distant event of horizontal transfer and retained as a pathogenicity island VSports手机版. The cag system is an Helicobacter-specific type IV secretion engine involved in cellular responses like induction of pedestals, secretion of IL-8, and phosphorylation of proteic targets. It has previously been reported that cocultivation of epithelial cells with Helicobacter pylori triggers signal transduction and tyrosine phosphorylation of a 145-kDa putative host cell protein. Herein, we demonstrate that this protein is not derived from the host but rather is the bacterial immunodominant antigen CagA, a virulence factor commonly expressed in peptic ulcer disease and thought to be an orphan of a specific biological function. Thus, CagA is delivered into the epithelial cells by the cag type IV secretion system where it is phosphorylated on tyrosine residues by an as yet unidentified host cell kinase and wired to eukaryotic signal transduction pathways and cytoskeletal plasticity. .
Figures



References
-
- Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Science. 1999;284:1328–1333. - PubMed
-
- Papini E, Satin B, Bucci C, de Bernard M, Telford J L, Manetti R, Rappuoli R, Zerial M, Montecucco C. EMBO J. 1997;16:15–24. - PMC (VSports手机版) - PubMed
-
- Papini E, Gottardi E, Satin B, de Bernard M, Massari P, Telford J, Rappuoli R, Sato S B, Montecucco C. J Med Microbiol. 1996;45:84–89. - PubMed
-
- Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, et al. Nature (London) 1999;397:176–180. - PubMed (VSports注册入口)
-
- Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, et al. Nature (London) 1997;388:539–547. - PubMed (V体育安卓版)
Publication types
- Actions (V体育官网入口)
MeSH terms
- VSports - Actions
- "V体育安卓版" Actions
- Actions (V体育ios版)
- Actions (VSports app下载)
- "V体育平台登录" Actions
- Actions (V体育2025版)
- V体育平台登录 - Actions
- "V体育官网入口" Actions
- V体育平台登录 - Actions
- VSports app下载 - Actions
"VSports最新版本" Substances
- Actions (VSports)
- VSports最新版本 - Actions
VSports app下载 - LinkOut - more resources
Full Text Sources

