"V体育官网入口" Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma
- PMID: 10587357
- PMCID: PMC2195739
- DOI: "V体育ios版" 10.1084/jem.190.11.1669
Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma
Abstract
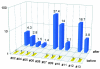
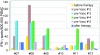
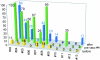
Dendritic cells (DCs) are considered to be promising adjuvants for inducing immunity to cancer. We used mature, monocyte-derived DCs to elicit resistance to malignant melanoma. The DCs were pulsed with Mage-3A1 tumor peptide and a recall antigen, tetanus toxoid or tuberculin. 11 far advanced stage IV melanoma patients, who were progressive despite standard chemotherapy, received five DC vaccinations at 14-d intervals VSports手机版. The first three vaccinations were administered into the skin, 3 x 10(6) DCs each subcutaneously and intradermally, followed by two intravenous injections of 6 x 10(6) and 12 x 10(6) DCs, respectively. Only minor (less than or equal to grade II) side effects were observed. Immunity to the recall antigen was boosted. Significant expansions of Mage-3A1-specific CD8(+) cytotoxic T lymphocyte (CTL) precursors were induced in 8/11 patients. Curiously, these immune responses often declined after the intravenous vaccinations. Regressions of individual metastases (skin, lymph node, lung, and liver) were evident in 6/11 patients. Resolution of skin metastases in two of the patients was accompanied by erythema and CD8(+) T cell infiltration, whereas nonregressing lesions lacked CD8(+) T cells as well as Mage-3 mRNA expression. This study proves the principle that DC "vaccines" can frequently expand tumor-specific CTLs and elicit regressions even in advanced cancer and, in addition, provides evidence for an active CD8(+) CTL-tumor cell interaction in situ as well as escape by lack of tumor antigen expression. .
Figures







VSports手机版 - References
-
- Schreiber H. Tumor Immunology. In: Paul W.E., editor. Fundamental Immunology. Lippincott-Raven Publishers; Philadelphia: 1999. pp. 1237–1270.
-
- Van den Eynde B.J., van der Bruggen P. T cell defined tumor antigens. Curr. Opin. Immunol. 1997;9:684–693. - PubMed (V体育安卓版)
-
- Pardoll D.M. Cancer vaccines Nat. Med. 4Suppl.1998. 525 531 - PubMed
-
- Romero P., Dunbar P.R., Valmori D., Pittet M., Ogg G.S., Rimoldi D., Chen J.L., Lienard D., Cerottini J.C., Cerundolo V. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J. Exp. Med. 1998;188:1641–1650. - PMC - PubMed
Publication types
MeSH terms
- "V体育ios版" Actions
- "VSports最新版本" Actions
- V体育2025版 - Actions
- Actions (V体育2025版)
- "VSports app下载" Actions
- Actions (VSports)
- "VSports手机版" Actions
- Actions (VSports注册入口)
- Actions (VSports app下载)
- Actions (VSports手机版)
Substances
- "V体育平台登录" Actions
- VSports注册入口 - Actions
- "V体育官网入口" Actions
- Actions (VSports)
- "V体育官网" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical (VSports注册入口)
Research Materials

