VSports手机版 - Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis
- PMID: 10557326
- PMCID: PMC23953
- DOI: 10.1073/pnas.96.23.13363
Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis
Abstract
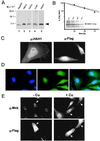
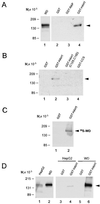
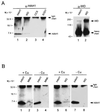
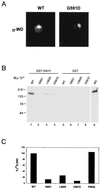
The delivery of copper to specific sites within the cell is mediated by distinct intracellular carrier proteins termed copper chaperones. Previous studies in Saccharomyces cerevisiae suggested that the human copper chaperone HAH1 may play a role in copper trafficking to the secretory pathway of the cell. In this current study, HAH1 was detected in lysates from multiple human cell lines and tissues as a single-chain protein distributed throughout the cytoplasm and nucleus. Studies with a glutathione S-transferase-HAH1 fusion protein demonstrated direct protein-protein interaction between HAH1 and the Wilson disease protein, which required the cysteine copper ligands in the amino terminus of HAH1. Consistent with these in vitro observations, coimmunoprecipitation experiments revealed that HAH1 interacts with both the Wilson and Menkes proteins in vivo and that this interaction depends on available copper. When these studies were repeated utilizing three disease-associated mutations in the amino terminus of the Wilson protein, a marked diminution in HAH1 interaction was observed, suggesting that impaired copper delivery by HAH1 constitutes the molecular basis of Wilson disease in patients harboring these mutations VSports手机版. Taken together, these data provide a mechanism for the function of HAH1 as a copper chaperone in mammalian cells and demonstrate that this protein is essential for copper homeostasis. .
Figures

V体育官网 - References
-
- Solomon E I, Lowery M D. Science. 1993;259:1575–1581. - PubMed (V体育官网)
-
- Harris Z L, Gitlin J D. Am J Clin Nutr. 1996;63:836S–841S. - PubMed
-
- Schaefer M, Gitlin J D. Am J Physiol. 1999;276:G311–G314. - PubMed
-
- Valentine J S, Gralla E B. Science. 1997;278:817–818. - V体育官网入口 - PubMed
Publication types
VSports注册入口 - MeSH terms
- "V体育官网入口" Actions
- Actions (VSports注册入口)
- Actions (V体育官网入口)
- Actions (V体育官网入口)
- "V体育官网" Actions
- Actions (VSports最新版本)
- V体育ios版 - Actions
Substances (VSports最新版本)
- Actions (VSports在线直播)
- V体育ios版 - Actions
- Actions (V体育官网)
- VSports手机版 - Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
- "VSports最新版本" Actions
Grants and funding
LinkOut - more resources (V体育平台登录)
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

