SnoN and Ski protooncoproteins are rapidly degraded in response to transforming growth factor beta signaling
- PMID: 10535941
- PMCID: PMC22943
- DOI: 10.1073/pnas.96.22.12442
SnoN and Ski protooncoproteins are rapidly degraded in response to transforming growth factor beta signaling
Abstract
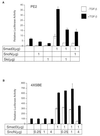
Transforming growth factor beta (TGF-beta) regulates a variety of physiologic processes, including growth inhibition, differentiation, and induction of apoptosis. Some TGF-beta-initiated signals are conveyed through Smad3; TGF-beta binding to its receptors induces phosphorylation of Smad3, which then migrates to the nucleus where it functions as a transcription factor. We describe here the association of Smad3 with the nuclear protooncogene protein SnoN VSports手机版. Overexpression of SnoN represses transcriptional activation by Smad3. Activation of TGF-beta signaling leads to rapid degradation of SnoN and, to a lesser extent, of the related Ski protein, and this degradation is likely mediated by cellular proteasomes. These results demonstrate the existence of a cascade of the TGF-beta signaling pathway, which, upon TGF-beta stimulation, leads to the destruction of protooncoproteins that antagonize the activation of the TGF-beta signaling. .
Figures


References (VSports注册入口)
-
- Hoodless P A, Wrana J L. Curr Top Microbiol Immunol. 1998;228:235–272. - PubMed
-
- Kingsley D M. Genes Dev. 1994;8:133–146. - "VSports在线直播" PubMed
-
- Massague J. Annu Rev Biochem. 1998;67:753–791. - PubMed
-
- Roberts A B, Flanders K C, Heine U I, Jakowlew S, Kondaiah P, Kim S J, Sporn M B. Philos Trans R Soc London B. 1990;327:145–154. - PubMed
-
- Massague J. Annu Rev Cell Biol. 1990;6:597–641. - PubMed
Publication types
MeSH terms
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- Actions (VSports在线直播)
- "VSports注册入口" Actions
- Actions (VSports手机版)
- Actions (VSports最新版本)
Substances
- Actions (V体育平台登录)
- "V体育ios版" Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- "VSports app下载" Actions
- "VSports app下载" Actions
- Actions (V体育ios版)
- "VSports app下载" Actions
- VSports最新版本 - Actions
- "VSports app下载" Actions
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources
VSports app下载 - Full Text Sources
Molecular Biology Databases
Research Materials (V体育官网入口)

