"V体育官网" The BH3 domain of Bcl-x(S) is required for inhibition of the antiapoptotic function of Bcl-x(L)
- PMID: 10490606
- PMCID: PMC84651
- DOI: 10.1128/MCB.19.10.6673
The BH3 domain of Bcl-x(S) is required for inhibition of the antiapoptotic function of Bcl-x(L)
Abstract
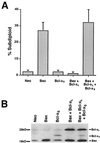
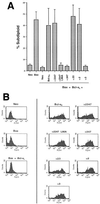
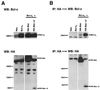
bcl-x is a member of the bcl-2 family of genes. The major protein product, Bcl-x(L), is a 233-amino-acid protein which has antiapoptotic properties. In contrast, one of the alternatively spliced transcripts of the bcl-x gene codes for the protein Bcl-x(S), which lacks 63 amino acids present in Bcl-x(L) and has proapoptotic activity VSports手机版. Unlike other proapoptotic Bcl-2 family members, such as Bax and Bak, Bcl-x(S) does not seem to induce cell death in the absence of an additional death signal. However, Bcl-x(S) does interfere with the ability of Bcl-x(L) to antagonize Bax-induced death in transiently transfected 293 cells. Mutational analysis of Bcl-x(S) was conducted to identify the domains necessary to mediate its proapoptotic phenotype. Deletion mutants of Bcl-x(S) which still contained an intact BH3 domain retained the ability to inhibit survival through antagonism of Bcl-x(L). Bcl-x(S) was able to form heterodimers with Bcl-x(L) in mammalian cells, and its ability to inhibit survival correlated with the ability to heterodimerize with Bcl-x(L). Deletion mutants of Bax and Bcl-2, which lacked BH1 and BH2 domains but contained a BH3 domain, were able to antagonize the survival effect conferred by Bcl-x(L). The results suggest that BH3 domains from both pro- and antiapoptotic Bcl-2 family members, while lacking an intrinsic ability to promote programmed cell death, can be potent inhibitors of Bcl-x(L) survival function. .
Figures



References
-
- Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nunez G, Thompson C B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. - V体育官网入口 - PubMed
-
- Chen G, Branton P E, Yang E, Korsmeyer S J, Shore G C. Adenovirus E1B 19-kDa death suppressor protein interacts with Bax but not with Bad. J Biol Chem. 1996;271:24221–24225. - PubMed
-
- Cheng E H-Y, Kirsch D G, Clem R J, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. - "VSports手机版" PubMed
-
- Clarke M F, Apel I J, Benedict M A, Eipers P G, Sumantran V, Gonzalez-Garcia M, Doedens M, Fukunaga N, Davidson B, Dick J E, et al. A recombinant bcl-xs adenovirus selectively induces apoptosis in cancer cells but not in normal bone marrow cells. Proc Natl Acad Sci USA. 1995;92:11024–11028. - PMC - PubMed
MeSH terms
- "V体育平台登录" Actions
- "VSports" Actions
- VSports最新版本 - Actions
- Actions (V体育ios版)
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- "V体育2025版" Actions
- V体育官网入口 - Actions
Substances
- Actions (VSports)
- V体育平台登录 - Actions
- V体育2025版 - Actions
- V体育2025版 - Actions
- V体育ios版 - Actions
LinkOut - more resources
Full Text Sources
Research Materials (V体育平台登录)
