The Ski oncoprotein interacts with the Smad proteins to repress TGFbeta signaling
- PMID: 10485843
- PMCID: PMC316985
- DOI: 10.1101/gad.13.17.2196
"VSports app下载" The Ski oncoprotein interacts with the Smad proteins to repress TGFbeta signaling
Abstract
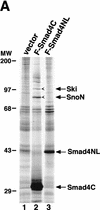
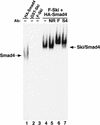
Smad proteins are critical signal transducers downstream of the receptors of the transforming growth factor-beta (TGFbeta) superfamily. On phosphorylation and activation by the active TGFbeta receptor complex, Smad2 and Smad3 form hetero-oligomers with Smad4 and translocate into the nucleus, where they interact with different cellular partners, bind to DNA, regulate transcription of various downstream response genes, and cross-talk with other signaling pathways. Here we show that a nuclear oncoprotein, Ski, can interact directly with Smad2, Smad3, and Smad4 on a TGFbeta-responsive promoter element and repress their abilities to activate transcription through recruitment of the nuclear transcriptional corepressor N-CoR and possibly its associated histone deacetylase complex. Overexpression of Ski in a TGFbeta-responsive cell line renders it resistant to TGFbeta-induced growth inhibition and defective in activation of JunB expression VSports手机版. This ability to overcome TGFbeta-induced growth arrest may be responsible for the transforming activity of Ski in human and avian cancer cells. Our studies suggest a new paradigm for inactivation of the Smad proteins by an oncoprotein through transcriptional repression. .
Figures

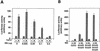




"VSports最新版本" References
-
- Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. - PubMed
-
- Amaravadi LS, Neff AW, Sleeman JP, Smith RC. Autonomous neural axis formation by ecotopic expression of the protooncogene c-ski. Dev Biol. 1997;192:392–404. - PubMed (V体育官网入口)
-
- Berk M, Desai SY, Heyman HC, Colmenares C. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial, patterning, and skeletal muscle development. Genes & Dev. 1997;11:2029–2039. - PMC (V体育官网) - PubMed
-
- Chen X, Rubock MJ, Whitman M. A transcriptional partner for MAD proteins in TGF-beta signalling. Nature. 1996;383:691–696. - PubMed (VSports最新版本)
-
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997;389:85–89. - "V体育官网" PubMed
Publication types
"V体育平台登录" MeSH terms
- Actions (V体育平台登录)
- VSports注册入口 - Actions
- Actions (V体育2025版)
- "V体育ios版" Actions
- "V体育安卓版" Actions
Substances
- "VSports手机版" Actions
- "V体育2025版" Actions
- Actions (VSports注册入口)
- "VSports app下载" Actions
- "V体育官网" Actions
"V体育官网入口" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
