Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene
- PMID: 10454544
- PMCID: "V体育官网入口" PMC84466
- DOI: "VSports在线直播" 10.1128/MCB.19.9.5969
Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene (VSports最新版本)
Abstract
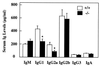
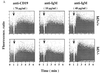
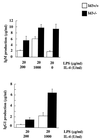
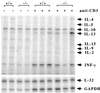
B-lymphocyte activation and proliferation induced by the B-cell receptor (BCR) signals are important steps in the initiation of humoral immune responses. How the BCR signals are translated by nuclear transcription factors into cell cycle progression is poorly understood. Id3 is an immediate-early gene responding to growth and mitogenic signals in many cell types including B cells. The primary function of the Id3 protein has been defined as that of inhibitor of basic-helix-loop-helix (bHLH) transcription factors. The interaction between Id3 and bHLH proteins, many of which are essential for cellular differentiation, has been proposed as a key regulatory event leading to cellular proliferation instead of differentiation. To further investigate the role of Id3 in tissue and embryo development and the mechanism of Id3-mediated growth regulation, we generated and analyzed Id3-deficient mice. While these mice display no overt abnormality in tissue and embryo development, their humoral immunity is compromised VSports手机版. The amounts of immunoglobulins produced in Id3-deficient mice immunized with a T-cell-dependent antigen and a type 2 T-cell-independent antigen are attenuated and severely impaired, respectively. Further analysis of lymphocytes isolated from Id3-deficient mice reveals a B-cell defect in their proliferation response to BCR cross-linking but not to lipopolysaccharide or a combination of BCR cross-linking and interleukin-4. Analyses of cultured lymphocytes also suggest involvement of Id3 in cytokine production in T cells and isotype switching in B cells. Finally, the proliferation defect in Id3-deficient B cells can be rescued by ectopic expression of Id1, a homologue of Id3. Taken together, these results define a necessary and specific role for Id3 in mediating signals from BCR to cell cycle progression during humoral immune responses. .
Figures








References
-
- Atherton G T, Travers H, Deed R, Norton J D. Regulation of cell differentiation in C2C12 myoblasts by the Id3 helix-loop-helix protein. Cell Growth Differ. 1996;7:1059–1066. - PubMed
-
- Bain G, Robanus Maandag E C, te Riele H P, Feeney A J, Sheehy A, Schlissel M, Shinton S A, Hardy R R, Murre C. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. - PubMed
-
- Bain G, Maandag E C, Izon D J, Amsen D, Kruisbeek A M, Weintraub B C, Krop I, Schlissel M S, Feeney A J, van Roon M, van der Valk M, te Riele H P J, Berns A, Murre C. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. - PubMed
-
- Barone M V, Pepperkok R, Peverali F A, Philipson L. Id proteins control growth induction in mammalian cells. Proc Natl Acad Sci USA. 1994;91:4985–4988. - PMC (VSports最新版本) - PubMed
-
- Benezra R. An intermolecular disulfide bond stabilizes E2A homodimers and is required for DNA binding at physiological temperatures. Cell. 1994;79:1057–1067. - V体育官网入口 - PubMed
Publication types
- Actions (VSports最新版本)
- V体育ios版 - Actions
MeSH terms
- Actions (VSports注册入口)
- VSports app下载 - Actions
- Actions (VSports app下载)
- "VSports在线直播" Actions
- VSports手机版 - Actions
- "V体育平台登录" Actions
- V体育官网 - Actions
- "VSports app下载" Actions
- "VSports在线直播" Actions
- Actions (VSports手机版)
- "VSports在线直播" Actions
- "VSports手机版" Actions
Substances
- Actions (V体育平台登录)
- Actions (VSports手机版)
- Actions (V体育平台登录)
- "VSports" Actions
- Actions (V体育2025版)
"V体育官网" Grants and funding
LinkOut - more resources
"V体育安卓版" Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
