Inhibition of Epstein-Barr virus replication by a benzimidazole L-riboside: novel antiviral mechanism of 5, 6-dichloro-2-(isopropylamino)-1-beta-L-ribofuranosyl-1H-benzimidazole
- PMID: 10438815
- PMCID: PMC104252
- DOI: 10.1128/JVI.73.9.7271-7277.1999
V体育2025版 - Inhibition of Epstein-Barr virus replication by a benzimidazole L-riboside: novel antiviral mechanism of 5, 6-dichloro-2-(isopropylamino)-1-beta-L-ribofuranosyl-1H-benzimidazole
Abstract
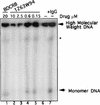
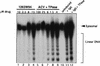
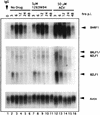
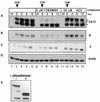
Although a number of antiviral drugs inhibit replication of Epstein-Barr virus (EBV) in cell culture, and acyclovir (ACV) suppresses replication in vivo, currently available drugs have not proven effective for treatment of EBV-associated diseases other than oral hairy leukoplakia. Benzimidazole riboside compounds represent a new class of antiviral compounds that are potent inhibitors of human cytomegalovirus (HCMV) replication but not of other herpesviruses. Here we characterize the effects of two compounds in this class against lytic replication of EBV induced in a Burkitt lymphoma cell line latently infected with EBV. We analyzed linear forms of EBV genomes, indicative of lytic replication, and episomal forms present in latently infected cells by terminal probe analysis followed by Southern blot hybridization as well as the high-molecular-weight unprocessed viral DNA by pulsed-field gel electrophoresis. D-Ribofuranosyl benzimidazole compounds that act as inhibitors of HCMV DNA maturation, including BDCRB (5, 6-dichloro-2-bromo-1-beta-D-ribofuranosyl-1H-benzimidazole), did not affect the accumulation of high-molecular-weight or monomeric forms of EBV DNA in the induced cells. In contrast, the generation of linear EBV DNA as well as precursor viral DNA was sensitive to the L-riboside 1263W94 [5, 6-dichloro-2-(isopropylamino)-1-beta-L-ribofuranosyl-1H-benzimidazole]. The 50% inhibitory concentration range for 1263W94 was 0. 15 to 1. 1 microM, compared with 10 microM for ACV. Thus, 1263W94 is a potent inhibitor of EBV. In addition, 1263W94 inhibited the phosphorylation and the accumulation of the essential EBV replicative cofactor, early antigen D VSports手机版. .
Figures




"V体育官网" References
-
- Ben-Bassat H, Goldblum N, Mitrani S, Goldblum T, Yoffey J M, Cohen M M, Bentwich Z, Ramot B, Klein E, Klein G. Establishment in continuous culture of a new type of lymphocyte from a ‘Burkitt like’ malignant lymphoma (line DG-75) Int J Cancer. 1977;19:27–33. - PubMed
-
- Biron, K. K. Unpublished data.
Publication types
- Actions (V体育官网)
MeSH terms
- "V体育ios版" Actions
- "VSports最新版本" Actions
- V体育平台登录 - Actions
- "VSports最新版本" Actions
- "V体育官网" Actions
- "V体育ios版" Actions
- VSports app下载 - Actions
- Actions (VSports app下载)
Substances
- "VSports最新版本" Actions
- "V体育官网" Actions
- "VSports最新版本" Actions
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources

