SAG, a novel zinc RING finger protein that protects cells from apoptosis induced by redox agents (VSports最新版本)
- PMID: 10082581
- PMCID: PMC84108
- DOI: 10.1128/MCB.19.4.3145
V体育ios版 - SAG, a novel zinc RING finger protein that protects cells from apoptosis induced by redox agents
Abstract
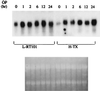
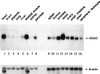
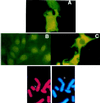
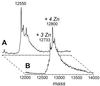
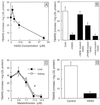
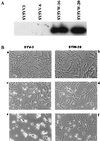
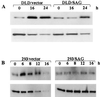
SAG (sensitive to apoptosis gene) was cloned as an inducible gene by 1,10-phenanthroline (OP), a redox-sensitive compound and an apoptosis inducer. SAG encodes a novel zinc RING finger protein that consists of 113 amino acids with a calculated molecular mass of 12. 6 kDa. SAG is highly conserved during evolution, with identities of 70% between human and Caenorhabditis elegans sequences and 55% between human and yeast sequences. In human tissues, SAG is ubiquitously expressed at high levels in skeletal muscles, heart, and testis. SAG is localized in both the cytoplasm and the nucleus of cells, and its gene was mapped to chromosome 3q22-24 VSports手机版. Bacterially expressed and purified human SAG binds to zinc and copper metal ions and prevents lipid peroxidation induced by copper or a free radical generator. When overexpressed in several human cell lines, SAG protects cells from apoptosis induced by redox agents (the metal chelator OP and zinc or copper metal ions). Mechanistically, SAG appears to inhibit and/or delay metal ion-induced cytochrome c release and caspase activation. Thus, SAG is a cellular protective molecule that appears to act as an antioxidant to inhibit apoptosis induced by metal ions and reactive oxygen species. .
VSports手机版 - Figures



References
-
- Abello P A, Fidler S A, Bulkley G B, Buchman T G. Antioxidants modulate induction of programmed endothelial cell death (apoptosis) by endotoxin. Arch Surg. 1994;129:134–141. - PubMed
-
- Adebodun F, Post J F. Role of intracellular free Ca(II) and Zn(II) in dexamethasone-induced apoptosis and dexamethasone resistance in human leukemic CEM cell lines. J Cell Physiol. 1995;163:80–86. - VSports app下载 - PubMed
-
- Allen R G, Balin A K. Oxidative influence in development and differentiation: an overview of a free radical theory of development. Free Radical Biol Med. 1989;6:631–661. - PubMed
-
- Auld D S. Use of chelating agents to inhibit enzymes. Methods Enzymol. 1988;158:110–114. - "V体育安卓版" PubMed
-
- Baker A, Payne C M, Briehl M M, Powis G. Thioredoxin, a gene found overexpressed in human cancer, inhibits apoptosis in vitro and in vivo. Cancer Res. 1997;57:5162–5167. - PubMed
Publication types
- VSports最新版本 - Actions
MeSH terms
- "V体育ios版" Actions
- Actions (VSports app下载)
- "VSports app下载" Actions
- Actions (V体育平台登录)
- VSports手机版 - Actions
- Actions (V体育平台登录)
- "V体育ios版" Actions
- "VSports app下载" Actions
- VSports - Actions
- Actions (V体育2025版)
- "VSports注册入口" Actions
- "V体育官网" Actions
"VSports app下载" Substances
- V体育平台登录 - Actions
- "VSports在线直播" Actions
- "V体育官网入口" Actions
- V体育ios版 - Actions
- "VSports app下载" Actions
- Actions (VSports手机版)
Associated data
- VSports - Actions
- Actions (VSports最新版本)
LinkOut - more resources
"V体育ios版" Full Text Sources
Other Literature Sources
Molecular Biology Databases
VSports手机版 - Miscellaneous
