Abstract
MYCN is a potential target for cancer immunotherapy by virtue of its overexpression in numerous human malignancies and its functional role in tumour progression. Here we show limited expression of MYCN in normal human tissues indicating that anti-MYCN immune responses are unlikely to cross react with self tissues. An HLA-A2 restricted ten amino acid peptide epitope from MYCN, VILKKATEYV, was used to stimulate cytotoxic T cell lines from the peripheral blood of normal blood donors, and from a patient with MYCN amplified neuroblastoma VSports最新版本. Strong and specific activity was seen against each MYCN overexpressing cell line and against autologous tumour cells. We generated two CTL clones capable of killing cells pulsed with as low as 0. 5 nM of VIL peptide. Therefore strong and specific immune responses against MYCN expressing tumours are possible in patients with the most common HLA class 1 type in the Caucasian population.
Keywords: MYCN, Immunotherapy, Dendritic cells, Peptide epitope, Cancer
Introduction
The rationale of cancer immunotherapy is the manipulation of the host immune system to develop potent, specific and sustained anti-tumour immune responses with minimal toxicity. In melanoma, successful elimination of established tumours has been reported following adoptive transfer of ex vivo expanded tumour infiltrating lymphocytes (TIL), identifying autologous T cells as a powerful potential therapeutic tool for metastatic and chemoresistant disease [1, 2]. Analysis of TIL in immunogenic tumours such as melanoma has led to the identification of natural tumour specific antigens VSports注册入口. However, in most cancers naturally occurring TIL either do not exist or have not been studied in detail and alternative methods of identifying tumour antigens are required, especially for poor prognosis tumours unresponsive to conventional therapy. One approach is the theoretical determination of putative tumour antigens, which will have the following three characteristics: high expression in the tumour, low expression in normal tissues, and a role in oncogenesis that implies that the tumour will not be able to escape immune detection through downregulation of expression of the antigen. This “candidate antigen” approach has been used successfully for a number of potential immunotherapy targets that lack evidence for natural immunogenicity for example, PSA [3], WT1 [4], CD45 [5], CD19 [6]. All these targets have limitations however because they are not required for oncogenesis and/or are also expressed in some normal tissues.
MYCN has previously been recognised as an attractive target for immunotherapy because it meets all the three above criteria [7, 8] V体育官网入口. The normal tissue expression of MYCN in murine development is seen in multiple tissues including heart, limb buds and neural tube [9]. At birth it is expressed in heart, lung, intestine, kidney and brain but this expression becomes downregulated over the first weeks of life [10–12]. This limited expression might also theoretically decrease the likelihood of immunological tolerance. MYCN is overexpressed, frequently as a consequence of genomic amplification, in a large number of malignancies including neuroblastoma, rhabdomyosarcoma, Wilms, retinoblastoma, astrocytoma, medulloblastoma and small cell lung cancer [13–18]. MYCN shares a high level of sequence and functional similarity with the product of the ubiquitously expressed c-myc oncogene [19]. Experimentally induced downregulation of MYC protein expression in tumour cells results in tumour regression in animal models highlighting the attractiveness of MYCN as a therapeutic target in cancer [20].
In this study we have confirmed the absence of MYCN expression in normal human adult tissue and have identified an immunogenic epitope of MYCN restricted to HLA-A2, the most common class 1 allele in the Caucassian population. Using this peptide epitope it is possible to expand T cell lines and clones with specific cytotoxicity against MYCN expressing tumours VSports在线直播. This proof of principle will allow the further evaluation of anti-MYCN T cell responses generated using alternate techniques for the treatment of MYCN dependent malignancies.
Materials and methods
T2 binding assays
Peptides were synthesised by Zinsser Analytic, UK. T2 cells were incubated overnight at 3 × 105 cells per well in a 96-well plate in RPMI media supplemented with 5% boiled FCS and serial dilutions of peptides, in the presence of 2 μg/ml β-2 microglobulin (SIGMA). Cells were washed in PBS and stained with FITC conjugated antibody specific for HLA-A2 (2 μg/ml; Becton Dickinson, UK) V体育2025版. After two wash steps with PBS, cells were analysed by flow cytometry.
PBMC from blood donors and patients
Fresh peripheral blood from healthy donors, were centrifuged over Ficoll-Hypaque to obtain peripheral blood mononuclear cells (PBMC) following Institutional Ethics Review Board approval. PBMCs and tumour samples were available from a patient with a relapsed MYCN expressing neuroblastoma who had been treated with autologous monocyte derived dendritic cells pulsed with autologous tumour lysate VSports.
Generation of human T cell lines and cloning
Unless indicated all cytokines and GM-CSF were purchased from PeproTech. Dendritic cells and CTL lines were generated as previously described [21]. Briefly, adherent cells (1. 5 × 106/well of six-well plate) were cultured in 10% AB serum, IL-4 (30 ng/ml), and GM-CSF (100 ng/ml) for 7 days, with replenishment on days 3 and 5. On day 6, DCs were matured with 10 μg/ml Keyhole Limpet Hemacyanin, (KLH, Calbiochem, Darmstadt, Germany), CD40L at 500 ng/ml (Biosource, UK), and prostaglandin E2 (Cambridge Laboratories, UK, 500 ng/ml) VSports app下载. CD8 T cells were stimulated two times at weekly intervals by autologous peptide (10 μM) pulsed DCs, and a further two stimulations using autologous CD40-L activated B cells as an alternative source of highly efficient antigen presenting cells [22].
On day 7 mature DCs were pulsed with 10 μM of peptide for 4 h, γ-irradiated and cocultured with autologous CD8+ T cells positively selected from PBMC using MACS technology (Miltenyi, Bergisch Gladbach, Germany). 1 × 106 CD8+ T cells were incubated with autologous mature, peptide pulsed DCs in the presence of IL-12 (20 U/ml) and IL-7 (10 ng/ml) with a ratio of T cells:DCs of 10:1. T cells were restimulated for a second week with autologous mature peptide pulsed DC in the presence of IL-12, IL-7 and IL-2. Two further weekly stimulations using autologous peptide-loaded (10 μM) CD40-activated B cells were carried out to maintain the specific T cell line. Autologous CD40-activated B cells were generated as previously described [22]. Briefly, CD40L stably transfected mouse fibroblast cells (t-CD40L-cells, from Dr. John Gordon) were γ-irradiated at 100 Gy, plated at 3. 5 × 105 cells/well in six-well plates in basal iscove’s medium + 10% FCS and incubated overnight. CD8 T cell depleted PBMC were added at 1–2 × 106 cells/ml in the presence of 5. 5 × 10−7 M Cyclosporin A (Sandoz Pharmaceutical) for 4 days. Fresh irradiated t-CD40L-cells were added every 4 days. B cells generated were 75% CD19 positive. T cells were harvested after four rounds of stimulation. T cells staining with both CD8 and a VIL; A2 specific pentamer were FACS sorted and were further cloned by the limiting dilution method (at 0. 4 and 1 cell/well) using γ-irradiated (25 Gy) allogeneic peripheral-blood leukocytes at 1 × 105 cells/well and irradiated B-LCL (75 Gy) at 1 × 103/well, in RPMI medium, containing 250 IU IL-2 and 1 μg/ml PHA V体育官网. After 12 days, growing T cell clones were screened by 51Cr cytotoxic killing and IFN-γ ELISPOT assay and subsequently expanded in RPMI medium.
Interferon-γ ELISPOT and chromium release
For ELISPOT assay, effector T cell line or clones were plated at 1 × 105 cells/well of a 96-well plate. Cells were co-cultured with target cells for 72 h in the presence of IL-2 (5 U/ml). T2 target cells were pulsed with peptides. Target cells were pulsed T2 cells, 293T transfected with empty vector or MYCN expressing plasmid, or tumour target cells. Background level was determined in wells containing effector cells in the presence of an irrelevant peptide or an irrelevant tumour lysate, or effector cells only.
In chromium release assays, CTL lines or clones were used as effector cells. Target cells were labelled with 100 μCi Na2 51CrO4 in cell culture medium containing 10% FCS for 60 min at 37°C. The cells were washed twice in culture medium. Targets were next pulsed with the specific or non-specific control peptides (0.03 nM–10 μM/ml) for 1 h, washed once in culture medium and re-suspended at 5 × 104 cells/ml. When IMR32 and SKNAS neuroblastoma tumour cell lines were used as targets, they were pre-incubated in the presence of IFN-γ (800 IU/ml) for 48 h followed by FACS analysis to confirm upregulation of HLA-A2 antigen expression prior to the chromium assay. Effector cells were added at different E:T ratios. After 4 h, 25 μl of the assay supernatant was placed into a 96-well Lumaplate (Packard). The plates were dried and radioactivity counted using a Microbeta scintillation counter (PerkinElmer). Percentage specific lysis was calculated as (Experimental release − spontaneous release/total release − spontaneous release) × 100.
V体育ios版 - Transient transfection, quantitative RT-PCR, immunoblot and cell lines
293T cells were transiently co-transfected with pBK-CMV or with pBK-CMV-MYCN in the presence of GFP expressing vector and orthinine decarboxylase promoter–luciferase reporter. Five micrograms of plasmid DNA was co-transfected into 107 293T cells in a 100-mm petri dish using Lipofectamine 2000 (Invitrogen, UK) in accordance with the manufacturer’s instructions. The transient transfection efficiency was more than 90%. Gene-specific primers/probes for the N terminus region of GAPDH and MYCN were purchased from Applied Biosystems. Relative quantitation was determined by the 2−DDct method (Applied Biosystems Users Bulletin 2). Neuroblastoma cell lines IMR32 and SKNAS were purchased from ATCC, rhabdomyosarcoma cells lines RH18, RH30 were originally a gift from Peter Houghton. Anti-MYCN antibody has been described previously [14].
Antibodies and pentamer staining (VSports)
For blocking assays, cells were incubated for 1 h at room temperature with human anti-CD8 mAb (clone G42-8, BD Bioscience Pharmingen), or with human anti-MHC-Class-I A, B and C mAb (clone W6/32). Tumour cell lines were stained with HLA-A2 mAb (BB.7.1, BD Bioscience Pharmingen). The PE-labelled Pentamer (Proimmune, Oxford, UK) was titred and used at the optimal concentration (5–10 μg/ml) to assay the VIL specific CD8+ T cells. For staining, 1 × 105 T cells were washed and re-suspended in pentamer staining buffer consisting of D-PBS, 0.1% NaN3 and 0.1% BSA. The cells were stained with 10 μl pentamer-PE for 20 min at RT, washed and then stained in buffer with 5 μl anti-human CD8-FITC (BD Biosciences) for 20 min at RT. The cells were washed in buffer and fixed in D-PBS and 1% paraformaldehyde (PFA), and were analysed using a Cyan flow cytometer (DAKO, Glostrup, Denmark). The pentamer-positive cells were analysed on a two-colour plot with Summit (DAKO, Glostrup, Denmark).
Results
MYCN is a suitable target for immunotherapy in cancer patients
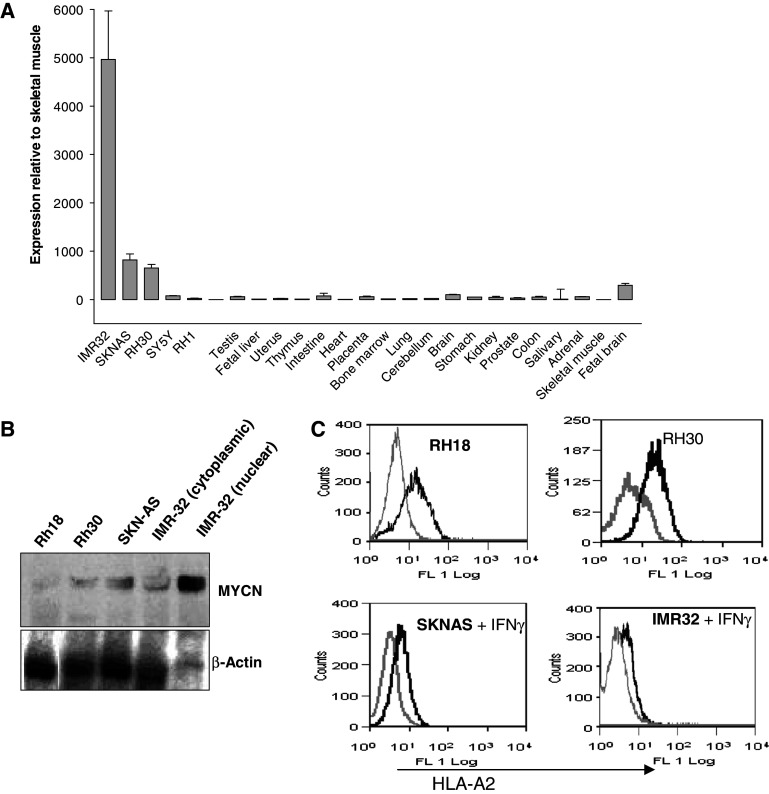
We investigated the relative expression of MYCN in human tumour cells and normal tissues. Quantitative RT-PCR indicated that MYCN is almost undetectable in a panel of normal adult human tissues. In contrast expression in MYCN amplified (IMR32) and non-amplified (SKNAS) neuroblastoma cells was 800- and 5,000-fold higher, respectively, than the levels seen in skeletal muscle (Fig. 1a). RH30 rhabdomyosarcoma cells with low level MYCN amplification expressed MYCN about 700-fold higher than skeletal muscle (Fig. 1a). Only fetal brain of the normal tissues showed levels comparable with the deregulated tumour cells. We confirmed that RT-PCR data corresponded with protein expression in the cell lines IMR32, SKNAS, RH30 and RH18, which were subsequently used as target cells in cytotoxicity assays (Fig. 1b). Our aim was to generate HLA-A2 restricted CTL responses directed against MYCN, and so we measured the presence of this common MHC class 1 molecule on the surface of this panel of potential target cells (Fig. 1c). Neuroblastoma cells IMR32 and SKNAS have low surface class I expression and these cells needed to be pre-incubated with IFN-γ to observe any HLA-A2 positive staining (data not shown).
Fig. 1.
Differential MYCN expression in normal and cancer tissues. a MYCN expression at the RNA level measured by quantitative RT-PCR in a panel of normal human tissues and some rhabdomyosarcoma and neuroblastoma cell lines. b Immunoblot analysis of MYCN expression in cells lines used in cytotoxicity assays. IMR32 lysate was derived separately from nuclear and cytoplasmic extracts and each IMR32 sample reflects an equivalent number of cells. c HLA-A2 epression as determined by flow cytometry; pale line is isotype control, thick line is HLA-A2 staining. IMR32 cell line was treated with IFN-γ (5,000 U/ml) for 24 h before staining
A novel HLA-A2 restricted peptide epitope from MYCN is immunogenic
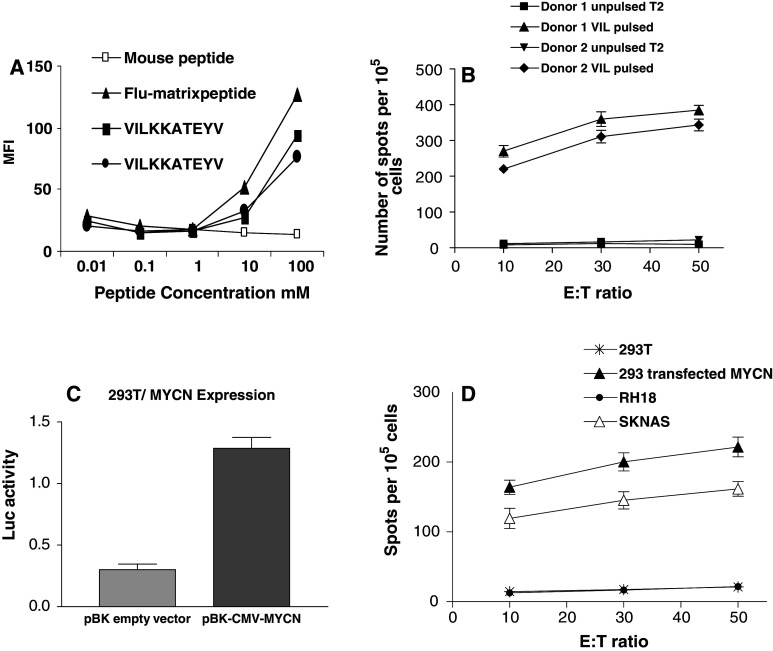
Because there is a several order of magnitude degree of upregulation of MYCN in tumours compared with normal tissues we reasoned that the cellular immune responses directed against tumours are unlikely to cross react with normal tissues to cause autoimmunity. Using two computational algorithms (SYTHPETHI and BIMAS) we identified the VILKKATEYV (VIL) sequence from human MYCN, predicted to bind HLA-A2. In T2 binding assays the VIL peptide showed favourable binding avidity compared with the immunodominant Flu matrix control peptide (Fig. 2a). A BLAST search revealed that the VIL epitope was not found in any other human protein sequence, including C-MYC.
Fig. 2.
T cell lines from two separate donors directed against the VIL epitope are capable of the specific recognition of MYCN expressing cells. a T2 binding assay in which duplicate VIL peptides were tested against positive (Flu marix) and negative (irrelevant mouse peptide) controls. b Anti-VIL T cell lines from two separate HLA-A2 positive donors are equally specifically reactive against VIL pulsed T2 cells in IFN-γ ELISPOT assay. Error bars reflect standard error of the mean of triplicate assays. c Transient transfection assay in which A2 positive 293T cells were cotransfected with MYCN and ornithine carboxylase promoter–luciferase reporter. MYCN activity in lysates is quantified in terms of relative light units following normalisation of the luciferase assay. Error bars are SEM of triplicates. d IFN-γ release following coculture of anti-VIL T cell lines with 293T target cells depicted in c or with MYCN positive SKNAS cells or MYCN negative RH18 cells
We next successfully generated CTL lines directed against VIL from two out of four normal HLA-A2 positive blood donors. In two of the donors, there was failure of T cell expansion. The protocol for CTL generation involved four stimulations at weekly intervals. The first two stimulations were with autologous DCs pulsed with VIL, and the last two with pulsed autologous B cells and irradiated allogeneic feeder cells. IL2 and IL7 were added for the first 2 weeks and IL2 and IL12 for the second 2 weeks. The VIL primed CTL lines from both normal donors showed high specific IFN-γ release following exposure to T2 cells pulsed with 10 μM of VIL peptide, but negligible IFN-γ release in the absence of peptide (Fig. 2b). To assess whether the T cell lines were also capable of recognising the VIL epitope following endogenous processing and presentation of MYCN on MHC class 1 molecules, we transfected 293T cells (HLA-A2 positive MYCN low) with MYCN, and observed a fivefold increase in MYCN protein function (Fig. 2c). The VIL CTL line from donor 1 showed specific IFN-γ release when co-cultured with these MYCN transfected 293T cells but not with vector transfected cells. Similar levels of IFN-γ release were observed following incubation of this T cell line with SKNAS neuroblastoma cells (HLA-A2 positive, MYCN expressing) but not with RH18 (HLA-A2 positive MYCN negative) (Fig. 2d). Therefore anti-VIL T cell lines are capable of a specific recognition of the VIL/HLA-A2 complex on the surface of MYCN expressing cells.
"V体育官网入口" T cell lines and clones derived from normal blood donors are capable of killing MYCN expressing cells
To determine whether T cell lines were capable of killing cancer cells expressing MYCN at physiological levels, we performed standard chromium release assays. T cell lines derived from both normal donors specifically killed HLA-A2+ (Fig. 1c) SKNAS neuroblastoma cells endogenously expressing MYCN, as well as killing 293T cell transfected with MYCN, and killing T2 cells pulsed with VIL peptide, whereas limited background killing activity was observed against unpulsed T2, untransfected 293 and against HLA-A2 positive but MYCN negative RH18 cells (Fig. 3a). The killing was mediated by cytotoxic T cells recognising peptide bound to MHC because killing of peptide pulsed T2 cells was blocked by both anti-MHC class 1 and anti-CD8 neutralising antibodies (Fig. 3b). Therefore CTL that can specifically recognise endogenously expressed and processed deregulated MYCN in tumour cells can be generated from the autologous repertoire of normal donors and will specifically lyse MYCN expressing tumour cells.
Fig. 3.
Anti-VIL T cell lines from normal donors can specifically kill MYCN expressing HLA-A2 positive target cells. a Standard chromium release assay at the E:T ratios indicated. b Specific killing of VIL pulsed T2 cells is inhibited by anti-MHC class 1 and anti-CD8 antibodies but not by equivalent concentration of isotype control antibody. Error bars indicate standard error of the mean of triplicate estimates of inhibition of a single T cell line
An anti-MYCN CTL line can be generated from a patient with advanced neuroblastoma
The childhood solid tumour neuroblastoma is a disease with high incidence of MYCN amplification, which is an adverse prognostic factor in this disease. To investigate whether MYCN directed effective killer cells can be generated from the autologous repertoire of patients with MYCN amplified neuroblastoma, we made use of blood and viable purified tumour cells from an HLA-A2 positive patient with metastatic neuroblastoma who had been pre-treated with intensive chemotherapy. A CTL line was generated from PBMC by stimulation with VIL pulsed autologous dendritic cells and B cells using the same protocol used for the normal blood donors. The resulting T cell line was tested for cytotoxicity. Specific lysis was observed against autologous tumour cells and IMR32 and RH30 tumour cell lines that were MYCN expressing and HLA-A2 positive (Fig. 1c for HLA-A2 expression), whereas no lysis was observed against HLA-A2 positive but MYCN negative RH18 cells (Fig. 4a). This patient from whom this CTL line had been generated had been previously treated on a dendritic cell vaccine phase 1 study in which autologous dendritic cells were pulsed will autologous tumour lysate and injected as an intradermal vaccination. We had available two blood samples from this patient taken at before vaccination and after just one DC vaccine. Stimulation of PBMC from this patient with autologous tumour lysate or with autologous DCs loaded with autologous tumour lysate induces a significant increase of IFN-γ secreting T cells following the vaccine administration indicating in vitro evidence of a successful anti-tumour immune response (Fig. 4b). We therefore addressed the hypothesis that anti-VIL response were a component of an anti-tumour response in this particular patient undergoing immunotherapy. PBMC pre- and post-vaccination were co-cultured with VIL alone or with VIL pulsed DCs in an ELISPOT assay, and showed a significant and specific induction of IFN-γ secreting T cells (Fig. 4c). This indicates that anti-VIL activity is a component of the anti-tumour immune response in this patient.
Fig. 4.
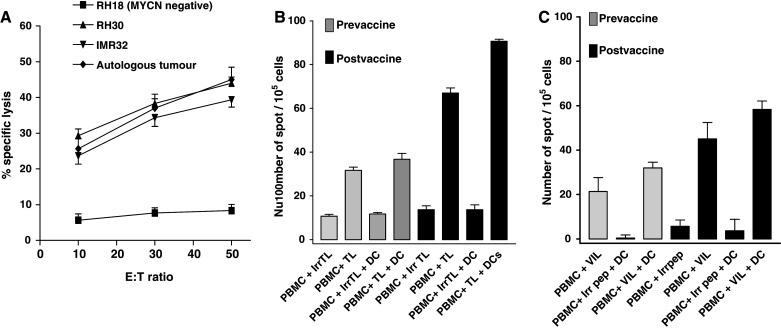
Anti-VIL is a component of an anti-tumour response in one patient with a MYCN expressing neuroblastoma. a CTL line derived from the peripheral blood of an HLA-A2 positive MYCN amplified neuroblastoma patient is capable of the specific lysis of autologous and MYCN positive tumour cell lines RH30 and IMR32. Error bars indicate standard error of the mean. b PBMC from this patient before and after DC/autologous lysate vaccination, were co-cultured with 20 μg/ml of autologous tumour lysate (TL) or irrelevant tumour lysate (irrTL, prepared from TC32, Ewing sarcoma HLA-A2+ tumour cell line) pulsed or not onto autologous DCs for 48 h. c 1 × 105 PBMC taken pre-vaccination, or post vaccination, were co-cultured with 10 μM VIL or an irrelevant peptide (KLTEARVQV peptide, a HLA-A2 restricted epitope derived from human PAX3 protein, Irr peptide), or co-cultured with 104 peptide pulsed DC. Data are means of triplicate estimations and error bars are SEM
Anti-MYCN CTL clones can kill MYCN expressing tumour cells in a HLA restricted manner
With the aim of generating specific clones, we FACS sorted an anti-VIL CTL line stained with specific VIL pentamer and CD8 (Fig. 5a) and cloned T cells by limiting dilution. We screened clones for killing following addition of T2 cells pulsed with VIL peptide at concentrations of 10 μM, by chromium release assay. Two clones (VIL/C1 and VIL/C2) from a total of 12 clones screened were found to exhibit specific killing at effector:target ratio as low as 5:1 and no killing of an irrelevant peptide pulsed T2 cells (Fig. 5b), and so these were further characterised by INF-γ ELISPOT assay to determine the avidity of the interaction with the VIL:A2 complex. The highest avidity clone (Clone VIL/C2) specifically induce IFN-γ secreting T cells when co-cultured with DCs pulsed with as little as 0.125–0.5 nM peptide (Fig. 5c) suggesting the possibility of generating high avidity TCR clones from VIL CTL lines. These could potentially be used for TCR gene cloning with a view to generating therapeutic immunotherapy vectors against MYCN.
Fig. 5.
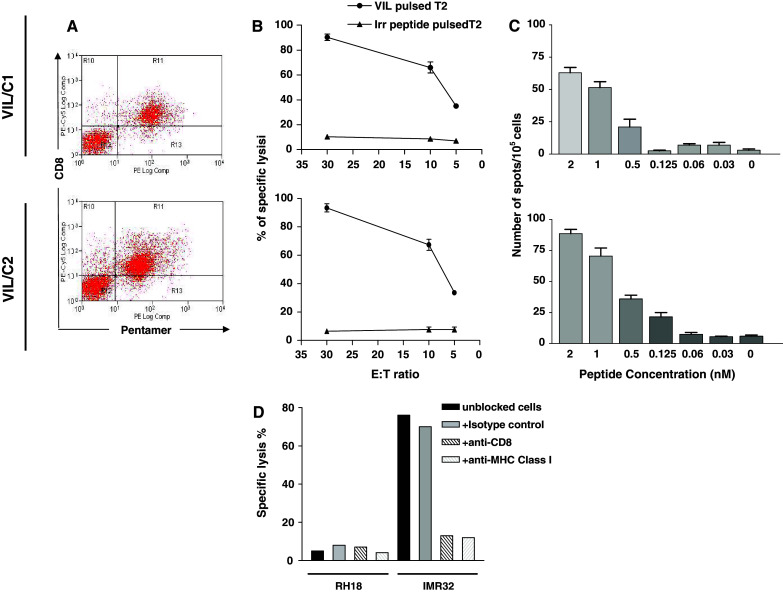
Specific CTL clones were raised against MYCN derived peptide; clone VIL/C1 is shown at the top and VIL/C2 is shown at the bottom. a Pentamer staining of CTL clone VIL/C1 and VIL/C2. b Killing of T2 cell pulsed with 10 μM of VIL peptide by specific clones VIL/C1 top and VIL/C2 bottom. c VIL peptide titration, T2 target cells were pulsed with different concentration of VIL epitope and co-culture with effector clones VIL/C1 top and VIL/C2 bottom at effector to target ratio of 10:1. Data are means of triplicate estimations and error bars are SEM. d Killing of IMR32 cell line by clone VIL/C2. IMR32 cells were pre-cultured for 48 h with IFN-γ prior to the assay, E:T ratio shown is 50:1
Discussion
We reasoned that MYCN is an excellent potential target for immunotherapy because we showed it to be virtually undetectable in normal tissues whereas MYCN over expression is seen in numerous adult cancers and childhood cancers including neuroblastoma and rhabdomyosarcoma. Most importantly, its oncogenic function and association with high-risk disease in neuroblastoma and rhabdomyosarcoma limit the likelihood of immune escape variants with downregulated MYCN. We hypothesised that its limited developmental expression might reduce the likelihood of tolerance in the T cell repertoire. We chose to start a search for epitopes from MYCN by looking for sequences predicted to have high affinity for HLA-A2 because it is the most common HLA type in Caucasians. Previous workers have identified an HLA-A1 derived MYCN peptide capable of inducing an anti-tumour immune response but HLA-A1 is a significantly less common allele [8]. Of the peptides we tested, only one was found to have affinity for HLA-A2 and so it was selected for CTL line generation from autologous T cells. Although CTL lines generated following stimulation with peptide pulsed antigen presenting cells are polyclonal there was still a high degree of specific killing observed indicating the possible presence of T cells within the line with high avidity. Short-term stimulation to generate T cell lines with specific killing activity is of interest for cancer immunotherapy because of three potential applications. Firstly expanded autologous T cells can be used in adoptive transfer in clinical protocols, and the successful generation of a T cell line from a heavily pre-treated patient is suggestive of potential feasibility of this approach. Secondly, ex vivo expanded pools of T cells can be used as a source of T cell clones expressing high avidity T cell receptors for gene therapy. Thirdly, peptides from MYCN could be used as cancer vaccines with or without pulsing onto dendritic cells.
Neuroblastoma is widely regarded to be a tumour with downregulated MHC class 1. However despite low levels of class1, the killing of both neuroblastoma cell lines and autologous tumour cells was comparable to that seen with transfected 293 cells. Moreover the killing activity of the T cell lines and clones was inhibited by anti-MHC class 1 and anti-CD8 molecules indicating a MHC restricted CTL activity. This suggests that MYCN over expressing neuroblastoma cells could be good targets for CTL mediated therapy despite being MHC low. The reason for this could be the obligate very high levels of MYCN typically seen in neuroblastoma cells and the upregulation of MHC to improve specific killing of those cells in the presence of IFN-γ. MYCN expression is observed additionally in a range of paediatric and adult cancers where its deregulation is thought to be a critical oncogenic event. The elucidation of its potential immunogenicity should stimulate interest in assessing anti-MYCN immune responses in a number of other cancer types.
Acknowledgments
This study was supported by grants from SPARKS, Cancer Research UK, and RICC.
V体育安卓版 - References
- 1.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [V体育ios版 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang KY. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst. 1997;89:293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 4.Gao L, Bellantuono I, Elsasser A, Marley SB, Gordon MY, Goldman JM, Stauss HJ. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–2203. [PubMed] [Google Scholar]
- 5.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 7.Morgenstern DA, Anderson J. MYCN deregulation as a potential target for novel therapies in rhabdomyosarcoma. Expert Rev Anticancer Ther. 2006;6:217–224. doi: 10.1586/14737140.6.2.217. ["VSports在线直播" DOI] [PubMed] [Google Scholar]
- 8.Sarkar AK, Nuchtern JG. Lysis of MYCN-amplified neuroblastoma cells by MYCN peptide-specific cytotoxic T lymphocytes. Cancer Res. 2000;60:1908–1913. [V体育官网 - PubMed] [Google Scholar]
- 9.Jakobovits A, Schwab M, Bishop JM, Martin GR. Expression of N-myc in teratocarcinoma stem cells and mouse embryos. Nature. 1985;318:188–191. doi: 10.1038/318188a0. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman KA, Yancopoulos GD, Collum RG, Smith RK, Kohl NE, Denis KA, Nau MM, Witte ON, Toran-Allerand D, Gee CE, et al. Differential expression of myc family genes during murine development. Nature. 1986;319:780–783. doi: 10.1038/319780a0. ["V体育官网" DOI] [PubMed] [Google Scholar]
- 11.Zimmerman K, Legouy E, Stewart V, Depinho R, Alt FW. Differential regulation of the N-myc gene in transfected cells and transgenic mice. Mol Cell Biol. 1990;10:2096–2103. doi: 10.1128/mcb.10.5.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semsei I, Ma SY, Cutler RG. Tissue and age specific expression of the myc proto-oncogene family throughout the life span of the C57BL/6J mouse strain. Oncogene. 1989;4:465–471. [PubMed] [Google Scholar]
- 13.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 14.Wong AJ, Ruppert JM, Eggleston J, Hamilton SR, Baylin SB, Vogelstein B. Gene amplification of c-myc and N-myc in small cell carcinoma of the lung. Science. 1986;233:461–464. doi: 10.1126/science.3014659. [DOI] [PubMed] [Google Scholar]
- 15.Williamson D, Lu YJ, Gordon T, Sciot R, Kelsey A, Fisher C, Poremba C, Anderson J, Pritchard-Jones K, Shipley J. Relationship between MYCN copy number and expression in rhabdomyosarcomas and correlation with adverse prognosis in the alveolar subtype. J Clin Oncol. 2005;23:880–888. doi: 10.1200/JCO.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 16.Garson JA, McIntyre PG, Kemshead JT. N-myc amplification in malignant astrocytoma. Lancet. 1985;2:718–719. doi: 10.1016/S0140-6736(85)92950-2. ["VSports" DOI] [PubMed] [Google Scholar]
- 17.Lee WH, Murphree AL, Benedict WF. Expression and amplification of the N-myc gene in primary retinoblastoma. Nature. 1984;309:458–460. doi: 10.1038/309458a0. [V体育2025版 - DOI] [PubMed] [Google Scholar]
- 18.Nisen PD, Zimmerman KA, Cotter SV, Gilbert F, Alt FW. Enhanced expression of the N-myc gene in Wilms’ tumors. Cancer Res. 1986;46:6217–6222. [PubMed] [Google Scholar]
- 19.Malynn BA, de Alboran IM, O’Hagan RC, Bronson R, Davidson L, DePinho RA, Alt FW. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 2000;14:1390–1399. [V体育官网入口 - PMC free article] [PubMed] [Google Scholar]
- 20.Pelengaris S, Littlewood T, Khan M, Elia G, Evan G. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol Cell. 1999;3:565–577. doi: 10.1016/S1097-2765(00)80350-0. ["V体育官网入口" DOI] [PubMed] [Google Scholar]
- 21.Himoudi N, Nabarro S, Yan M, Gilmour K, Thrasher AJ, Anderson J. Development of anti-PAX3 immune responses; a target for cancer immunotherapy. Cancer Immunol Immunother. 2007;56:1381–1395. doi: 10.1007/s00262-007-0294-3. [VSports最新版本 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, Delgado JC, Gribben JG, Nadler LM. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–2765. doi: 10.1172/JCI119822. [VSports最新版本 - DOI] [PMC free article] [PubMed] [Google Scholar]