Abstract
Background
A community-based randomized trial was conducted in Costa Rica to evaluate the HPV-16/18 AS04-adjuvanted vaccine (NCT00128661) VSports最新版本. The primary objective was to evaluate efficacy of the vaccine to prevent cervical intraepithelial neoplasia 2 or more severe disease (CIN2+) associated with incident HPV-16/18 cervical infections. Secondary objectives were to evaluate efficacy against CIN2+ associated with incident cervical infection by any oncogenic HPVs and to evaluate duration of protection against incident cervical infection with HPV-16/18. Vaccine safety and immunogenicity over the 4-year follow-up were also evaluated.
Methods
We randomized (3,727 HPV arm; 3,739 Control arm), vaccinated (HPV-16/18 or Hepatitis A) and followed (median 53. 8 months) 7,466 healthy women aged 18-25 years. 5,312 women (2,635 HPV arm; 2,677 Control arm) were included in the according to protocol analysis for efficacy. The full cohort was evaluated for safety V体育平台登录. Immunogenicity was considered on a subset of 354 (HPV-16) and 379 (HPV-18) women. HPV type was assessed by PCR on cytology specimens. Immunogenicity was assessed using ELISA and inhibition enzyme immunoassays. Disease outcomes were histologically confirmed. Vaccine efficacy and 95% confidence intervals (95%CI) were computed.
"VSports app下载" Results
Vaccine efficacy was 89. 8% (95% CI: 39. 5 - 99. 5; N=11 events total) against HPV-16/18 associated CIN2+, 59 VSports注册入口. 9% (95% CI: 20. 7 - 80. 8; N=39 events total) against CIN2+ associated with non-HPV-16/18 oncogenic HPVs and 61. 4% (95% CI: 29. 5-79. 8; N=51 events total) against CIN2+ irrespective of HPV type. The vaccine had an acceptable safety profile and induced robust and long-lasting antibody responses.
Conclusions
Our findings confirm the high efficacy and immunogenicity of the HPV-16/18 vaccine against incident HPV infections and cervical disease associated with HPV-16/18 and other oncogenic HPV types. These results will serve as a benchmark to which we can compare future findings from ongoing extended follow-up of participants in the Costa Rica trial V体育官网入口.
"V体育ios版" Trial Registration
Registered with clinicaltrials.gov: NCT00128661
Keywords: Human papillomaviruses, Cervical neoplasia, vaccination, prevention, clinical trial
Introduction
Three programs launched in the 2000s evaluated prophylactic virus-like particle (VLP) human papillomavirus (HPV) vaccines [1-3]. Two of these programs were led by manufacturing companies, Merck Pharmaceuticals and GlaxoSmithKline Biologicals, who licensed the HPV-VLP technology and developed vaccines to prevent cervical cancers caused by HPV-16 and HPV-18, two HPV types that account for up to 70% of cervical cancers worldwide V体育2025版. Results from trials by these companies demonstrated that the vaccines have an acceptable safety profile and are highly effective for the prevention of HPV infections and lesions associated with vaccine types, and in some instances to additional, related types. As a result, both vaccines are licensed for use in adolescents and young adults in many countries [4-10].
The third program initiated pre-licensure was a community-based trial in Costa Rica (NCT00128661) sponsored by the US National Cancer Institute (NCI) that utilized the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®, hereafter referred to as the HPV-16/18 vaccine) provided to NCI by GlaxoSmithKline Biologicals [11]. This trial was designed to evaluate the efficacy, safety, impact and immune mechanisms associated with HPV vaccination, and to extend natural history studies to vaccinated groups. To date, results from the Costa Rica HPV-16/18 Vaccine Trial (CVT) have shown that 1) the vaccine is highly effective at preventing new persistent infections with HPV-16/18[12], 2) the vaccine confers partial protection against HPV types phylogenetically related to HPV-16/18 [13], 3) the vaccine does not help treat infections [14], 4) fewer than 3 doses of the vaccine appear to protect as well as the full 3-dose series for at least 4 years against persistent HPV-16/18 infections [15], 5) there are indications that the vaccine protects against HPV infection at the anus and oral cavity [16, 17], 6) the vaccine does not impact overall rates of pregnancies/pregnancy outcomes [18], 7) the impact of vaccination declines with increasing age at vaccination [12], 8) the initial impact of young adult vaccination on colposcopy referral/treatment rates in well-screened populations are modest [19], 9) within the same age group, levels of antibodies achieved long-term following two doses (0 and 6 months) of the vaccine are high and only slightly lower than those observed after three doses and antibodies achieved long-term following one dose of the vaccine are lower than those observed with 3 doses but stable [20], 10) vaccination induces cross-neutralizing potential in sera of vaccinees [21], and that 11) modest antibody levels generated by natural HPV infection provide partial protection against re-infection [22] VSports.
We now extend those findings by presenting results from the blinded analysis conducted at the end of the first four years of follow-up. These results focus on the according to protocol (ATP) efficacy findings submitted to the FDA under BB-IND #7920; separate submissions focus on findings from intent-to-treat and naïve analyses from our trial [12, 23] VSports app下载.
Materials and Methods
"V体育官网" Design, Subjects, Procedures, and Testing
This analysis presents a double-blind randomized controlled trial of an HPV-16/18 vaccine among healthy women 18-25 years old. The study was approved by the Institutional Review Boards in Costa Rica and the US. Detailed methods have been published [11]. In brief, potential participants from a census were invited between June 2004 and December 2005. Eligible women who agreed to participate (N=7,466; estimated to provide >80% power to observe expected differences between arms) were randomized with equal chance to the HPV-16/18 (HPV arm) or Hepatitis A vaccine (Control arm), offered in three doses over approximately six months. Blinding to arm assignment was maintained throughout the 48-month follow-up and until the analytic datafile was frozen.
At enrollment, a pelvic exam was performed on sexually experienced women. Exfoliated cells were collected for cytology, HPV DNA, and other tests. At the 6-month visit, women were asked to provide a self-collected cervical specimen for HPV testing. Blood was collected from participants. Each participant was scheduled for annual follow-up examinations (median follow-up time = 53.8 months; inter-quartile range: 50.5 - 57.0), at which time a pelvic examination was performed on sexually active women, and exfoliated cells and blood were collected. On a pre-defined subset, an additional visit approximately one month following the last vaccine dose was performed where blood was collected for immunological assessment.
Cytology was classified using the Bethesda system. Women with low-grade squamous intraepithelial lesions (LSIL) or HPV positive atypical squamous cells of undetermined significance (ASC-US) were followed semi-annually. The colposcopy referral algorithm used in our trial parallels that used for the PATRICIA trial [6]. Specifically, a repeat LSIL/HPV positive ASC-US, an ASC-US-rule out high-grade SIL (ASC-H), high-grade squamous intraepithelial lesions or more severe disease (HSIL+), or glandular abnormalities prompted colposcopy and treatment as needed [11].
HPV testing using the Hybrid Capture 2 test was performed on enrollment specimens plus specimens from women with an ASC-US cytology during follow-up for clinical management [11]. Broad spectrum PCR-based HPV DNA testing was performed on specimens based on amplification and broad spectrum probe hybridization using the SPF10 HPV DNA enzyme immunoassay system followed by typing using the LiPA25 version 1 line detection system and HPV-16 and -18 type specific testing [11]. Enzyme-linked immunosorbent assay (ELISA) was used to detect and quantify IgG antibodies against HPV-16 and -18 in the subset of women selected for the extra visit one month after the last vaccination dose [4, 24]. Immunogenicity was also assessed by a V5/J4 monoclonal antibody inhibition enzyme immunoassay (EIA), which in contrast to the ELISA detects specific neutralizing epitopes [24, 25].
Objectives
The primary objective was to evaluate efficacy of the vaccine to prevent cervical intraepithelial neoplasia 2 or more severe disease (CIN2+) associated with incident (post dose 3) HPV-16/18 cervical infections. Secondary objectives were to evaluate efficacy to prevent CIN2+ associated with incident cervical infection by any oncogenic HPV type and to evaluate the duration of protection conferred by the vaccine against incident cervical infection with HPV-16/18. Vaccine safety and immunogenicity over the 4-year follow-up were also evaluated.
Analytical Cohorts
The cohort for efficacy analyses included subjects who received three doses within protocol-defined windows, whose timing between doses was respected (21–90 days between doses 1 and 2; 90–210 days between doses 2 and 3), who were HPV DNA negative at Months 0 and 6 for the HPV type considered in the analysis, who did not have a biopsy or treatment (Loop Electrosurgical Excisional Procedure) during the vaccination phase, for whom there was no investigational new drug safety report during the vaccination period, and who otherwise complied with the protocol during the vaccination period (Figure 1). The cohort for safety was defined as subjects who received at least one dose of vaccine and therefore represents the intention to treat cohort (N=7,466). The cohort for immunogenicity was defined as subjects included in the immunogenicity subcohort who met the criteria defined for the efficacy cohort above and whose timing between the third vaccine dose and the extra visit was 30–60 days (N=354 women for HPV-16 analysis; N=379 for HPV-18 analysis).
Figure 1. Consort Diagram - Analytic Cohort for Efficacy Analyses.
Outcomes
The primary outcome for efficacy was defined as histopathologically confirmed CIN2+ associated with HPV-16/18 cervical infection detected by PCR in the cervical cytology specimen that led to colposcopy referral. Final histological diagnosis was defined based on blinded review by a Costa Rican and a US pathologist, with blinded review by a third pathologist in instances where the first two reviewers disagreed [11]. In secondary efficacy analyses, we evaluated histopathologically confirmed CIN2+ associated with non-HPV-16/18 and any oncogenic HPV cervical infections (HPV types 16,18,31,33,35,39,45,51,52,56,58,59,68/73) detected by PCR in the cervical cytology specimen that led to colposcopy referral, and time to incident infection with HPV-16/18 cervical infections. In exploratory efficacy analyses, an alternative (referred to hereafter as “exploratory” to distinguish it from the a-priori definition described above) definition of HPV type attribution to CIN2+ lesions was used that considered evidence of HPV persistence preceding referral to colposcopy when attributing HPV types to lesions in instances when >1 HPV type was present in the cervical cytology specimen that led to colposcopy referral. We also evaluated histopathologically confirmed CIN2+, irrespective of HPV type, in an analysis that considered outcomes that occurred in the absence of HPV during the vaccination period.
For safety analyses, solicited local and general adverse events (AEs) within 60 minutes after vaccination (all subjects) or from Day 3-6 post-vaccination (10% random subset) were evaluated. Unsolicited AEs, serious adverse events (SAEs), and pregnancies/pregnancy outcomes were documented throughout the 4-year study period. Impact of vaccination on pregnancies/pregnancy losses was reported on separately [18] and is not considered here because limited new blinded information on pregnancies around vaccination was accrued after the initial report.
For immunogenicity analyses, we evaluated presence and level of HPV-16 and HPV-18 antibodies by ELISA and by HPV-16 V5 and HPV-18 J4 monoclonal antibody inhibition EIA measured during the vaccination period, at one month after the last vaccination, and at annual visits thereafter in the subjects enrolled into the immunogenicity cohort.
Statistical Analysis
Vaccine efficacy (VE), defined as the percentage reduction in an endpoint due to the vaccine, was estimated as the complement of the ratio of the attack rates (risk ratio) in the HPV and control arms. The attack rate was calculated as the percentage of women who experienced the endpoint. The complement of the 95% confidence interval (95% CI) for the risk ratio was used to calculate the CI for the VE estimates. The difference between the attack rates in the two arms was used to assess rate reductions. The CI for the difference was calculated using the conditional exact test. Separate analyses were conducted for HPV-16/18, all oncogenic HPV types combined, all oncogenic HPV types combined excluding HPV-16/18, individual HPV types, and irrespective of HPV type.
The proportion of subjects with at least one SAE classified by International Classification of Diseases Version 10 during the study is presented by study group. Similar information is presented for grade 3 (severe) SAEs and for SAEs classified by the local investigator as possibly related to vaccination. We report separately the proportion of subjects with at least one reported autoimmune AE, neurological AE or death.
Seropositivity rates and Geometric Mean Titers (GMTs) with 95% CIs were calculated. When calculating GMTs, antibody titers below the assay cut-off were given a value of half the cut-off.
"V体育官网" Results
Participants in the HPV and Control arms of the trial and included in the ATP cohort for efficacy were comparable with respect to age, clinic, sexual behavior and HPV-16/18 serology and DNA results at entry (Supplemental Table 1).
Number of CIN2+ events, rates and efficacy are presented in Table 1. Efficacy against incident HPV-16/18 associated CIN2+ was 89.8% (95% CI = 39.5–99.5; Rate Reduction = 3.4/1,000 women) using our a-priori algorithm for HPV type attribution and 88.7% (95% CI = 31.3–99.5; Rate Reduction = 3.0/1,000 women) using the alternative (exploratory) definition that considers viral persistence when making HPV type attribution. A total of 11 HPV-16/18 associated CIN2+ events were observed using our a-priori definition; 10 were CIN2 and one was a CIN3. The single HPV-16/18 CIN2+ event in the HPV arm occurred in a participant who at entry had antibodies against both HPV-16 and HPV-18, and evidence (by DNA test) of infection with a non-oncogenic HPV type (HPV-66), and who was positive (by DNA test) for HPV-16 and -45 11 months after enrollment and diagnosed with CIN3 15 months after enrollment. Efficacy estimates against CIN2+ associated with non-HPV-16/18 oncogenic HPV types were 59.9% (a-priori definition) and 78.7% (exploratory definition). The breakdown of HPV types detected by arm is summarized in Figures 2a (a-priori definition) and 2b (exploratory definition). Efficacy estimates irrespective of HPV type were 61.4% (95% CI = 29.5–79.8; Rate Reduction = 8.4/1,000 women; N = 37 in Control arm and 14 in HPV arm) by our a-priori and 75.3% (95% CI = 48.1–89.3; Rate Reduction = 9.2/1,000 women; N = 33 in Control arm and 8 in HPV arm) by our exploratory definition of incident outcomes. Results for individual oncogenic HPV types are summarized in Supplemental Tables 2a-b.
Table 1. Vaccine Efficacy Against CIN2+ Outcomes - ATP Cohort for Efficacy - Costa Rica HPV-16/18 Vaccine Trial (CVT)a.
| HPV Type | Arm | Women in ATP cohort, N | Women with CIN2+ events, n | Rate (per 1000 women) | Rate Reduction (95% CIa) | Efficacy, % (95% CIa) |
|---|---|---|---|---|---|---|
| HPV-16/18 | HPV | 2,635 | 1 | 0.4 | ||
| Control | 2,677 | 10 | 3.7 | 3.4 (1.0, 4.1) | 89.8 (39.5, 99.5) | |
| HPV-16/18 (Strict) | HPV | 2,635 | 1 | 0.4 | ||
| Control | 2,677 | 9 | 3.4 | 3.0 (0.7, 3.7) | 88.7 (31.3, 99.5) | |
| Non-HPV-16/18 Oncogenic | HPV | 2,643 | 11 | 4.2 | ||
| Control | 2,697 | 28 | 10.4 | 6.2 (1.7, 9.8) | 59.9 (20.7, 80.8) | |
| Non-HPV-16/18 Oncogenic (Exploratory) | HPV | 2,643 | 5 | 1.9 | ||
| Control | 2,697 | 24 | 8.9 | 7.0 (3.3, 9.3) | 78.7 (47.1, 92.8) | |
| Oncogenic HPV | HPV | 2,643 | 11 | 4.2 | ||
| Control | 2,697 | 33 | 12.2 | 8.1 (3.4, 11.7) | 66 (34.0, 83.5) | |
| Oncogenic HPV (Exploratory) | HPV | 2,643 | 5 | 1.9 | ||
| Control | 2,697 | 29 | 10.8 | 8.9 (5.1, 11.2) | 82.4 (57.0, 94.0) |
CIN2+ = Cervical intraepithelial neoplasia 2 or more severe disease;
ATP = According to protocol; 95% CI = 95% confidence interval.
Figure 2A. Distribution of CIN2+ Outcomes by HPV Type (a-priori) and Vaccination Arm – According to Protocol Cohort for Efficacy – Costa Rica HPV-16/18 Vaccine Triala.
a CIN2+ = Cervical intraepithelial lesion or more severe disease; HPV type attribution for a-priori outcome defined based on HPV type(s) detected in the cytology specimen that led to colposcopy referral.
b Only non-oncogenic incident HPV types were detected for three women, positivity for uncharacterized HPV types was observed for two women, and no HPV was detectable for two women.
Figure 2B. Distribution of CIN2+ Outcomes by HPV Type (exploratory) and Vaccination Arm – According to Protocol Cohort for Efficacy – Costa Rica HPV-16/18 Vaccine Triala.
a CIN2+ = Cervical intraepithelial lesion or more severe disease; HPV type attribution for exploratory outcome defined considering evidence of HPV persistence preceding colposcopy when multiple HPV types were present in the cytology specimen that led to colposcopy referral.
b Only non-oncogenic incident HPV types were detected for three women, positivity for uncharacterized HPV types was observed for two women, and no HPV was detectable for two women.
Efficacy against incident HPV-16/18 infections during the study was 79.5% (95% CI = 74.0–84.0; Rate Reduction = 115/1,000 women) (Table 2). Efficacy in this group of young adults was lowest in the first year of follow-up (57.1%; 95% CI = 33.2–73.0) and higher in subsequent years (82.6% in year 4+; 95% CI = 73.0–89.2).
Table 2. Vaccine Efficacy Against Incident HPV-16/18 Detection by Time Since First Vaccination - ATP Cohort for Efficacy - Costa Rica HPV-16/18 Vaccine Trial (CVT)a.
| Time | Arm | Women in ATP cohort, N | Women with HPV-16/18 events, n | Rate (per 1000 women) | Rate Reduction (95% CIa) | Efficacy, % (95% CIa) |
|---|---|---|---|---|---|---|
| Overall | HPV | 2,635 | 78 | 29.6 | ||
| Control | 2,677 | 387 | 144.6 | 115.0 (102.3, 126.1) | 79.5 (74.0, 84.0) | |
| Year 1 | HPV | 2,380 | 27 | 11.3 | ||
| Control | 2,420 | 64 | 26.4 | 15.1 (7.5, 21.7) | 57.1 (33.2, 73.0) | |
| Year 2 | HPV | 2,313 | 18 | 7.8 | ||
| Control | 2,349 | 117 | 49.8 | 42.0 (34.5, 47.8) | 84.4 (74.8, 90.7) | |
| Year 3 | HPV | 2,232 | 11 | 4.9 | ||
| Control | 2,166 | 88 | 40.6 | 35.7 (29.1, 40.3) | 87.9 (78.0, 93.8) | |
| Year 4+ | HPV | 2,421 | 22 | 9.1 | ||
| Control | 2,261 | 118 | 52.2 | 43.1 (35.0, 49.5) | 82.6 (73.0, 89.2) |
ATP = According to protocol;
95% CI = 95% confidence interval.
Safety findings are summarized in Table 3. Rates of solicited local and general AEs were comparable in the two arms in the hour following vaccination. The rate of local solicited AEs within 3-6 days following any vaccination was higher among those in the HPV arm (53.7% for all; 1.8% for grade 3 AEs) compared to the Control arm (19.9% for all; 0.0% for grade 3 AEs). Unsolicited AEs reported in the month following any vaccination were comparable between arms. The proportion of participants with SAEs, SAEs possibly related to vaccination, medically significant conditions, new-onset chronic diseases, autoimmune AEs, neurological AEs, and deaths were comparable between arms. All but 12 SAEs possibly related to vaccination were pregnancy related [18]. For the 12 remaining SAEs possibly related to vaccination, 7 occurred in the HPV arm (1 Crohn's disease, 1 ulcerative colitis, 1 rheumatoid arthritis, 1 haematuria, 1 thyrotoxicosis, 1 excessive and frequent menstruation with irregular cycle, and 1 somatoform autonomic dysfunction) and 5 in the Control arm (2 anaphylactic shock events, 1 generalized skin eruption, 1 acute appendicitis, and 1 unspecified abnormalities of gait/mobility). The 43 autoimmune events were equally distributed across arms (22 in HPV arm; 21 in Control arm) and were due to goitre (8 in HPV arm; 9 in Control arm), rheumatoid arthritis (4 in HPV arm; 6 in Control arm), inflammatory bowel disease (3 in HPV arm including 1 Crohn's disease; 2 in Control arm), systemic lupus erythematosus (2 in HPV arm; 1 in Control arm), insulin-dependent diabetes mellitus (1 in HPV arm; 1 in Control arm) and other conditions (4 in HPV arm; 2 in Control arm). The 15 deaths observed were equally distributed across arms (8 in HPV arm; 7 in Control arm) and were due to suicides (4 in Control arm), automobile accidents (1 in HPV arm; 2 in Control arm), physical assault (2 in HPV arm), cancer (1 in HPV arm; 1 in Control arm), Crohn's disease (1 in HPV arm), systemic lupus erythematosus (1 in HPV arm), HIV-associated conditions (1 in HPV arm), and acute myocardial infarction (1 in HPV arm).
"V体育平台登录" Table 3. Summary of Safety Outcomes - Total Vaccinated Cohorta Costa Rica HPV-16/18 Vaccine Trial (CVT).
| HPV Arm | Control Arm | |||
|---|---|---|---|---|
| n | % | n | % | |
| Within 60 Minutes Following Any Vaccination (N=3,727 HPV & 3,739 Control Arms) | ||||
| Any Solicited Adverse Event | ||||
| All | 2,534 | 68.0% | 2,519 | 67.4% |
| Grade 3 | 32 | 0.9% | 27 | 0.7% |
| Solicited Local Adverse Eventsb | ||||
| All | 1,918 | 51.5% | 1,902 | 50.9% |
| Grade 3 | 25 | 0.7% | 22 | 0.6% |
| Solicited General Adverse Eventsc | ||||
| All | 1,580 | 42.4% | 1,543 | 41.3% |
| Grade 3 | 8 | 0.2% | 6 | 0.2% |
| Within Day 3-6 Following Any Vaccination (N=380 HPV & 376 Control Arms; 10% sample) | ||||
| Any Solicited Adverse Event | ||||
| All | 358 | 94.2% | 339 | 90.2% |
| Grade 3 | 11 | 2.9% | 2 | 0.5% |
| Solicited Local Adverse Eventsb | ||||
| All | 204 | 53.7% | 75 | 19.9% |
| Grade 3 | 7 | 1.8% | 0 | 0.0% |
| Solicited General Adverse Eventsc | ||||
| All | 344 | 90.5% | 335 | 89.1% |
| Grade 3 | 4 | 1.1% | 2 | 0.5% |
| Within 30 Days Following Any Vaccination (N=3,727 HPV & 3,739 Control Arms) | ||||
| Unsolicited Adverse Events | ||||
| All | 1,638 | 43.9% | 1,536 | 41.1% |
| Grade 3 | 34 | 0.9% | 30 | 0.8% |
| Serious Adverse Events During Entire Study Period (N=3,727 HPV & 3,739 Control Arms) | ||||
| Any Serious Adverse Events | 912 | 24.5% | 891 | 23.8% |
| Serious Adverse Events Possibly Related to Vaccination | 53 | 1.4% | 39 | 1.0% |
| Medically Significant Conditionsd | 744 | 20.0% | 739 | 19.8% |
| New-onset Chronic Diseased | 383 | 10.3% | 417 | 11.2% |
| Autoimmune Adverse Eventsd | 22 | 0.6% | 21 | 0.6% |
| Neurological Adverse Eventsd | 626 | 16.8% | 591 | 15.8% |
| Deaths | 8 | 0.2% | 7 | 0.2% |
Four participants who erroneously received both vaccine types were assigned to their original randomization arm (1 HPV arm; 3 control arm). These individuals reported no Serious adverse events.
Solicited local adverse events included pain, redness and swelling.
Solicited general adverse events included fatigue, myalgia, arthralgia, GI symptoms, headache, rash, urticaria, and fever.
Medically significant conditions were defined as grade 3 (severe) serious adverse events. As described previously [36], all adverse events reported during the trial were compared with a pre-defined list of potential chronic diseases derived from the Medical Dictionary for Regulatory Activities. Determination of whether a chronic disease was of new onset was based on blinded review of the reported symptoms and the subject's pre-vaccination medical history. A separate list, restricted to potential autoimmune events (e.g. systemic lupus erythematosus, thyroiditis), which excluded allergy-related events or isolated signs and symptoms and events not considered to be autoimmune in origin, was used to identify new onset autoimmune diseases among events identified as new onset chronic diseases. Neurologic adverse events are all preferred terms belonging to the system organ class ‘nervous system disorders’.
Immunogenicity results are summarized in Figures 3a-d. GMTs peaked at one month following last dose, declined thereafter and remained relatively stable beyond 12-24 months post-vaccination. By ELISA, we observed that 100% of vaccinated participants were seropositive against HPV-16 and HPV-18 after three doses and remained seropositive at the end of the 4-year follow-up period. By EIA, we observed that 100% and 99.5% of vaccinated participants were seropositive against HPV-16(V5) and HPV-18(J4), respectively, after three doses. At the end of the 4-year follow-up period, 92.3% and 45.8% of vaccinated participants remained seropositive against HPV-16(V5) and HPV-18(J4), respectively.
Figure 3A. Kinetics of HPV-16 Antibody Response by Vaccination Arma. (ELISA Assay) – Immunogenicity Subcohort – Costa Rica HPV-16/18 Vaccine Trial (CVT).

a HPV Arm plotted irrespective of entry serostatus. Control Arm plotted separately by entry serostatus.
Figure 3D. Kinetics of HPV-18 Antibody Response by Vaccination Arm*. (J4 - EIA Assay) – Immunogenicity Subcohort – Costa Rica HPV-16/18 Vaccine Trial (CVT).
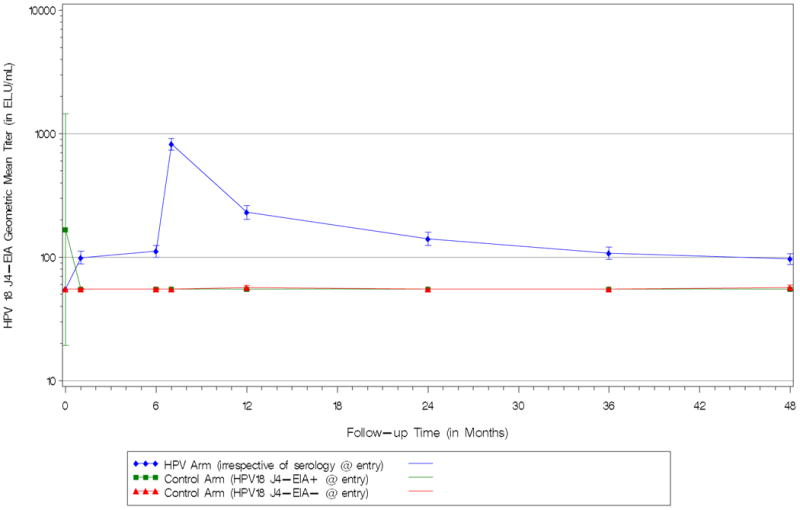
a HPV Arm plotted irrespective of entry serostatus. Control Arm plotted separately by entry serostatus
Discussion
This report summarizes results from the final ATP analysis of the NCI-sponsored CVT under GlaxoSmithKline Biologicals' FDA-BB-IND-7920. Our results confirm the high efficacy of VLP-based vaccines against incident CIN2+ associated with HPV-16/18 [4-10]. It is reassuring that high efficacy against infections and lesions associated with the HPV types in the vaccine formulation has now been reported for VLP-based vaccines from multiple trials conducted in different populations, despite differences in study methodology [4-10, 26, 27] (Table 4). Furthermore, our report is consistent with previous results suggesting that vaccination with the HPV-16/18 vaccine might confer partial protection against some oncogenic HPV types not included in the vaccine formulation [28]. We observed 60% efficacy against CIN2+ associated with incident oncogenic HPV infections with types other than HPV-16/18, an effect that increased to near 80% when we considered evidence of HPV persistence preceding referral to colposcopy. Although limited by small numbers, our findings suggest some efficacy (point estimates ranging from 42%-100%) for all HPV types phylogenetically related to HPV-16 (A9 species – including HPV types 31,33,35,52,58). For HPV types phylogenetically related to HPV-18 (A7 species – including HPV types 39,45,59,68), evidence was mixed, with suggestion for efficacy against HPV-68 (which in our testing system was indistinguishable from non-oncogenic HPV-73) but not for other types related to HPV-18. Finally, when CIN2+ cases were examined irrespective of HPV type, we observed over 60% efficacy, an effect that increased to >75% when our exploratory criteria were used to define incident outcomes. It is important to note that such estimates of overall efficacy are likely to be population specific and to vary depending on the proportion of infections in the population attributable to vaccine types, non-vaccine HPV types for which there is cross-protection, and non-vaccine HPV types for which there is no cross-protection. In fact, vaccine efficacy against non-vaccine types or irrespective of HPV type reported from phase III randomized clinical trials to date have varied considerably as summarized in Table 4. It is not fully understood to what extent these observed differences are due to differences in study design and analysis (e.g., differences in colposcopy algorithm, sensitivity/specificity of HPV assays, and analytical cohorts evaluated), chance (95% confidence intervals tend to overlap), population differences (e.g., differences in relative distribution of non-vaccine HPV types in different study populations), or vaccine differences (i.e., real differences in cross protection between the bivalent and quadrivalent vaccines). In a recent evaluation of this issue, we have noted that differences observed in efficacy estimates between FUTURE I/II and PATRICIA are likely explained by a combination of these various factors [23].
Table 4. Summary of Findings from Randomized Clinical Trials of Prophylactic Virus-Like Particle Human Papillomavirus Vaccines.
| Bivalent vaccine trials (HPV-16/18 AS04-adjuvanted vaccine) | Quadrivalent vaccine trials (HPV-6/11/16/18 vaccine) | |||||||
|---|---|---|---|---|---|---|---|---|
| Costa Rica Vaccine Trial (CVT) Phase III; NCT00128661 |
Japan Efficacy Trial [26] Phase II; NCT00316693/NCT00929526 |
PApilloma TRIal against Cancer In young Adults (PATRICIA) [6,28] Phase III; NCT00122681 |
Pooled analysis from 2-3 studies [9,37-39]: – 007: phase II; NCT n/a – 013 (FUTURE I): phase III; NCT00092521 – 015 (FUTURE II): phase III; NCT00092534 |
|||||
| Countries | Community-based trial (Guanacaste; Costa Rica) | Japan | Multi-country trial (Australia, Belgium, Brazil, Canada, Finland, Germany, Italy, Mexico, Philippines, Spain, Taiwan, Thailand, UK, and USA). | Multi-country trials (Australia, Austria, Brazil, Canada, Colombia, Czech Republic, Denmark, Finland, Germany, Hong Kong, Iceland, Italy, Mexico, New Zealand, Norway, Peru, Poland, Puerto Rico, Russia, Singapore, Sweden, Thailand, UK, USA) | ||||
| Age | 18–25 years | 20–25 years | 15–25 years | 16–26 years | ||||
| Total enrolled subjects | 7,466 | 1,046 | 18,729 | 18,174 (protocols 007, 013 and 015) | ||||
| Analytical cohort | ATP cohort for efficacy: received 3 doses within protocol defined windows, HPV DNA negative for the HPV type under consideration at Months 0 and 6, no biopsy or treatment (Loop Electrosurgical Excisional Procedure) during the vaccination phase, no protocol violation | ATP cohort for efficacy: received 3 doses within protocol defined windows, HPV DNA negative for the HPV type under consideration at Months 0 and 6, seronegative for the HPV type under consideration at Month 0, normal or low-grade cytology at Month 0, no protocol violation | Per-protocol: received 3 doses within 1 year, PCR negative and seronegative to HPV-6, HPV-11, HPV-16, or HPV-18 at enrolment, remained PCR negative to the same vaccine HPV type (s), to which they were naïve at enrolment, through 1 month post-dose 3, no protocol violation | |||||
| Endpoints | CIN2+: CIN2, CIN3, adenocarcinoma in situ or invasive carcinoma | CIN2+: CIN2, CIN3, adenocarcinoma in situ or invasive carcinoma | CIN2+: CIN2, CIN3, adenocarcinoma in situ or invasive carcinoma | CIN2 or worse | ||||
| Follow-up | 48 months post-dose 1 | 48 months post-dose 1 | 48 months post-dose 1 | 42 months post-dose 1 | ||||
| Efficacy estimates | ||||||||
| HPV-16/18 types | Current Manuscript | Konno et al. 2014 [26] | Lehtinen et al. 2012 [6] | Kjaer et al. 2009 [37] | ||||
| Vaccine | Control | Vaccine | Control | Vaccine | Control | Vaccine | Control | |
| N | 2,635 | 2,677 | 406 | 404 | 7,338 | 7,305 | 7,864 | 7,865 |
| Cases | 1 | 10 | 0 | 5 | 5 | 97 | 2 | 110 |
| Efficacy (95% CI) | 88.7% (31.3, 99.5)a | 100% (-8.0, 100) | 94.9% (87.7, 98.4) | 98.2% (93.3, 99.8)b | ||||
| Non-vaccine oncogenic types | HPV-31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -68/73 Current Manuscript | HPV-31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -68/73 [26] | HPV-31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -66, -68/73 [28] | HPV-31, -33, -35, -39, -45, -51, -52, -56, -58, -59 [38,39] | ||||
| Vaccine | Control | Vaccine | Control | Vaccine | Control | Vaccine | Control | |
| N | 2,643 | 2,697 | 444 | 435 | 8,067 | 8,047 | 4,616 | 4,680 |
| Cases | 5 | 24 | 4 | 12 | 88 | 165 | 62 | 93 |
| Efficacy (95% CI) | 78.7% (47.1, 92.8)a | 67.7% (-6.6, 92.4)c | 46.8% (30.7, 59.4) | 32.5% (6.0, 51.9)d | ||||
| Irrespective of HPV | Current Manuscript | Konno et al., 2014 [26] | Lehtinen et al., 2012 [6] | Munoz et al., 2010 [9] | ||||
| Vaccine | Control | Vaccine | Control | Vaccine | Control | Vaccine | Control | |
| N | 2,643 | 2,697 | 254 | 251 | 5466 | 5642 | 4,616 | 4,680 |
| Cases | 8 | 33 | 3 | 11 | 61 | 172 | 77 | 136 |
| Efficacy (95% CI) | 75.3% (48.1, 89.3)a | 73.9% (1.1, 95.3)e | 64.9% (52.7, 74.2)e | 42.7% (23.7, 57.3)d | ||||
Vaccine efficacy using exploratory definition described in the Methods section
Vaccine efficacy against HPV-6/11/16/18-related types for the quadrivalent vaccine
Vaccine efficacy in the ATP cohort for efficacy, regardless of baseline serostatus
Vaccine efficacy restricted to subjects who received ≥1 vaccine dose and, at enrolment, were HPV DNA negative for vaccine and nonvaccine types (31, 33, 35, 39, 45, 51, 52, 56, 58 and 59), seronegative for HPV-6, -11, -16 and -18, and had normal cytology
Vaccine efficacy in the TVC-naive cohort, i.e. women who received ≥1 vaccine dose and, at baseline, were HPV DNA negative for vaccine and nonvaccine types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68/73), seronegative for HPV-16 and -18, and had normal cytology
ATP: according-to-protocol; CI: Confidence Interval; CIN2+: cervical intraepithelial neoplasia 2 or more severe disease; n/a: not available; N: number of women in each arm considered in the analysis; TVC: total vaccinated cohort
We saw no evidence of waning efficacy during the study period. When we evaluated efficacy against HPV-16/18 infection over time, high efficacy (>80%) was observed in years 2-4+ and the lowest efficacy estimate was observed in the first year of follow-up (57%). The high efficacy observed in the out years is consistent with evidence of long-term protection up to 8.4 years (HPV-16/18 vaccine) and 5 years (HPV-6/11/16/18 vaccine) in the pharmaceutical trials [29, 30]. We interpret the somewhat reduced efficacy in year 1 as suggestive that some outcomes might have resulted from undetected infections present before vaccination in our group of largely sexually experienced women [12].
The safety and immunogenicity profile of VLP-based vaccine have been evaluated in large-scale trials and results suggest that that vaccine has an acceptable safety profile, is generally well tolerated, and induces a robust and sustained immune responses [7, 30-35]. Safety results from our trial are consistent with these previous reports. Similarly, consistent with previous reports, we noted robust antibody levels (measured by ELISA) following vaccination that persisted throughout the four years of follow-up – 100% of participants evaluated were seropositive against HPV-16 and HPV-18 at the end of follow-up. This is consistent with the high clinical efficacy observed. By the EIA inhibition assay that targets neutralizing epitopes for HPV-16 and HPV-18, we also observed robust responses following vaccination. These responses were measurable after four years for nearly all participants evaluated for HPV-16 (92.3%) and for roughly half of participants evaluated for HPV-18 (45.8%). Since efficacy remained high throughout the four years of follow-up for both HPV-16/18, the fact that about half of the vaccinees sero-reverted to HPV-18 by the EIA assay suggests that protective levels are lower than the minimum detectable level by the assay or that antibodies against additional epitopes can also be protective.
Limitations of our trial include the modest number of CIN2+ events among women naïve to specific HPV types during the vaccination period, which limited our ability to evaluate efficacy against individual HPV types other than HPV-16/18 and against CIN3+. Our study size also limited the ability to evaluate efficacy against lesions by time.
A distinguishing characteristic of our trial is its community-based design; we enrolled women from a well-defined area based on a census [11]. As a result, our trial represents a unique large-scale community-level trial conducted pre-licensure and affords an opportunity for follow-up studies to address many questions of interest. These include questions regarding long-term safety, immunogenicity and efficacy; natural history of infections in vaccinated women and the impact of vaccination on cervical disease associated with non-vaccine HPV types; the impact of vaccination on screening; and the utility of novel screening tools in vaccinated populations. The results presented herein serve as a benchmark to help interpret results from some of these planned efforts.
Our findings provide additional independent evidence of the efficacy, immunogenicity and safety of the HPV-16/18 vaccine for prevention of HPV infections and cervical cancer precursor lesions in previously unexposed women and further support the establishment of vaccination programs that target individuals prior to exposure.
Supplementary Material
Figure 3B. Kinetics of HPV-18 Antibody Response by Vaccination Arma. (ELISA Assay) – Immunogenicity Subcohort – Costa Rica HPV-16/18 Vaccine Trial (CVT). (VSports注册入口)

a HPV Arm plotted irrespective of entry serostatus. Control Arm plotted separately by entry serostatus.
Figure 3C. Kinetics of HPV-16 Antibody Response by Vaccination Arma. (V5 – EIA Assay) – Immunogenicity Subcohort – Costa Rica HPV-16/18 Vaccine Trial (CVT).

a HPV Arm plotted irrespective of entry serostatus. Control Arm plotted separately by entry serostatus.
Highlights.
A community-based trial of the HPV-16/18 vaccine was conducted among 7,466 women.
Vaccine efficacy was 89.8% for HPV-16/18 CIN2+ and 61.4% for any CIN2+.
The vaccine had an acceptable safety profile and induced robust antibody responses.
Results confirm the safety, immunogenicity and efficacy of the HPV-16/18 vaccine.
Acknowledgments
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women's Health, and done with the support from the Ministry of Health of Costa Rica. Vaccine was provided for our trial by GlaxoSmithKline Biologicals, under a Clinical Trials Agreement with the NCI. GlaxoSmithKline Biologicals also provided support for aspects of the trial associated with regulatory submission needs of the company under US Food and Drug Administration BB-IND 7920. JTS and DRL report that they are named inventors on US Government-owned HPV vaccine patents that are licensed to GlaxoSmithKline and Merck. They are entitled to limited royalties as specified by federal law. Jérôme Leemans, publications manager at Keyrus Biopharma (Belgium), coordinated the review/development process for this manuscript on behalf of GlaxoSmithKline Vaccines.
Abbreviations
- ATP
according to protocol
- AE
adverse event
- CI
confidence interval
- CIN2+
cervical intraepithelial neoplasia 2 or more severe disease
- CVT
Costa Rica HPV-16/18 Vaccine Trial
- GMT
geometric mean titer
- HPV
human papillomavirus
- DEIA
SPF10 HPV DNA enzyme immunoassay
- EIA
inhibition enzyme immunoassay
- ELISA
enzyme-linked immunosorbent assay
- NCI
National Cancer Institute
- SAE
serious adverse event
- VE
vaccine efficacy
- VLP
virus-like particle
Footnotes
Note: Cervarix is a registered trade mark of the GlaxoSmithKline group of companies.
Investigators in the Costa Rica Vaccine Trial (CVT) group: Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica—Mario Alfaro (cytopathologist), M. Concepción Bratti (co-investigator), Bernal Cortés (specimen and repository manager), Albert Espinoza (head, coding and data entry), Yenory Estrada (pharmacist), Paula González (co-investigator), Diego Guillén (pathologist), Rolando Herrero1 (co-principal investigator), Silvia E. Jiménez (trial coordinator), Jorge Morales (colposcopist), Lidia Ana Morera (head study nurse), Carolina Porras (co-investigator), Ana Cecilia Rodríguez (co-investigator), Luis Villegas (colposcopist).
University of Costa Rica, San José, Costa Rica—Enrique Freer (director, HPV diagnostics laboratory), José Bonilla (head, HPV immunology laboratory).
United States National Cancer Institute, Bethesda, MD, USA—Allan Hildesheim (co-principal investigator & NCI co-project officer), Aimée R. Kreimer (co-investigator), Douglas R. Lowy (DRL; HPV virologist), Nora Macklin (trial coordinator), Mark Schiffman (medical monitor & NCI co-project officer), John T. Schiller (JTS; HPV virologist), Mark Sherman (QC pathologist), Diane Solomon (medical monitor & QC pathologist), Sholom Wacholder (statistician).
SAIC, NCI-Frederick, Frederick, MD, UDA—Ligia Pinto (head, HPV immunology laboratory), Troy Kemp (immunologist).
Georgetown University, Washington, DC, USA—Mary Sidawy (histopathologist),
DDL Diagnostic Laboratory, Netherlands—Wim Quint (virologist, HPV DNA testing), Leen-Jan van Doorn (HPV DNA testing).
Present address: Prevention and Implementation Group, International Agency for Research on Cancer, World Health Organization, 150 Cours Albert Thomas, 69372, Lyon, France.
Conflicts of interest: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf. F.S., G.C. and G.D. are employees of the GlaxoSmithKline group of companies. G.D. and F.S. receive stock options/restricted shares from the GlaxoSmithKline group of companies, and G.D. has previously received patent royalties from Wyeth Vacines. The other authors declare that they have no conflicts of interest. The NCI receives licensing fees for HPV vaccines.
Author contribution: A.H. (NCI principal investigator), S.W. (NCI statistician) and R.H. (Costa Rica principal investigator) were responsible for the design and conduct of the study. From GlaxoSmithKline Vaccines, G.D. contributed to discussions regarding trial design and conduct. G.C. contributed towards data analyses and interpretation, and prepared the statistical analysis report submitted to the FDA. F.S. and G.D. critically reviewed the study report in close collaboration with NCI and Costa Rica co-principal investigators. A.H. wrote the manuscript, and all other authors reviewed and commented on the initial and subsequent drafts. All authors had full access to the data and gave final approval before submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sholom Wacholder, Email: wacholds@qiuluzeuv.cn.
Gregory Catteau, Email: GREGORY.X.CATTEAU@qiuluzeuv.cn.
Frank Struyf, Email: FRANK.STRUYF@qiuluzeuv.cn.
Gary Dubin, Email: Gary.O.Dubin@qiuluzeuv.cn.
Rolando Herrero, Email: HerreroR@qiuluzeuv.cn.
References
- 1.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. New Eng J Med. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 2.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 3.Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 viruslike particle vaccine. J Natl Cancer Inst. 2001;93:284–92. doi: 10.1093/jnci/93.4.284. [DOI (VSports app下载)] [PubMed] [Google Scholar]
- 4.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 5.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 6.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 7.GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–85. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 8.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. New Eng J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI (V体育官网入口)] [PubMed] [Google Scholar]
- 9.Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102:325–39. doi: 10.1093/jnci/djp534. ["VSports手机版" DOI] [PubMed] [Google Scholar]
- 10.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. New Eng J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 11.Herrero R, Hildesheim A, Rodriguez AC, Wacholder S, Bratti C, Solomon D, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26:4795–808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrero R, Wacholder S, Rodriguez AC, Solomon D, Gonzalez P, Kreimer AR, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011;1:408–19. doi: 10.1158/2159-8290.CD-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safaeian M, Kemp TJ, Pan DY, Porras C, Rodriguez AC, Schiffman M, et al. Cross-protective vaccine efficacy of the bivalent HPV vaccine against HPV31 is associated with humoral immune responses: Results from the Costa Rica Vaccine Trial. Hum Vaccin Immunother. 2013;9:1399–406. doi: 10.4161/hv.24340. [DOI (VSports)] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–53. doi: 10.1001/jama.298.7.743. [V体育2025版 - DOI] [PubMed] [Google Scholar]
- 15.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8:e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreimer AR, Gonzalez P, Katki HA, Porras C, Schiffman M, Rodriguez AC, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12:862–70. doi: 10.1016/S1470-2045(11)70213-3. ["V体育平台登录" DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wacholder S, Chen BE, Wilcox A, Macones G, Gonzalez P, Befano B, et al. Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials. BMJ. 2010;340:c712. doi: 10.1136/bmj.c712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez AC, Solomon D, Herrero R, Hildesheim A, Gonzalez P, Wacholder S, et al. Impact of human papillomavirus vaccination on cervical cytology screening, colposcopy, and treatment. Am J Epidemiol. 2013;178:752–60. doi: 10.1093/aje/kwt047. [V体育官网 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, et al. Durable Antibody Responses Following One Dose of the Bivalent Human Papillomavirus L1 Virus-Like Particle Vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res. 2013;6:1242–50. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemp TJ, Safaeian M, Hildesheim A, Pan Y, Penrose KJ, Porras C, et al. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix(®) Vaccine. 2012;31:165–70. doi: 10.1016/j.vaccine.2012.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102:1653–62. doi: 10.1093/jnci/djq384. [DOI (V体育安卓版)] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhs KA, Porras C, Schiller JT, Rodriguez AC, Schiffman M, Gonzalez P, et al. Differing human papillomavirus serological and DNA criteria may affect vaccine efficacy estimates. Am J Epidemiol, under revision. doi: 10.1093/aje/kwu168. ["VSports手机版" DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4:425–34. doi: 10.4161/hv.4.6.6912. ["VSports手机版" DOI] [PubMed] [Google Scholar]
- 25.Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–49. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Konno R, Yoshikawa H, Okutani M, Quint W, Suryakiran PV, Lin L, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young Japanese women: Open follow-up of a randomized clinical trial up to four years post-vaccination. Hum Vaccin Immunother. 2014 doi: 10.4161/hv.28712. (Epub ahead of print); http://dx.doi.org/10.4161/hv.28712 (VSports最新版本). [DOI] [PMC free article] [PubMed]
- 27.Zhu FC, Chen W, Hu YM, Hong Y, Li J, Zhang X, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: Results from a randomised controlled trial. Int J Canc. 2014 doi: 10.1002/ijc.28897. (Epub ahead of print); http://onlinelibrary.wiley.com/doi/10.1002/ijc.28897/abstract. [DOI] [PMC free article] [PubMed]
- 28.Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 29.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–66. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, et al. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother. 2012;8:390–7. doi: 10.4161/hv.18865. [DOI] [PubMed] [Google Scholar]
- 31.Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, et al. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin. 2009;5:332–40. doi: 10.4161/hv.5.5.7211. [DOI] [PubMed] [Google Scholar]
- 32.Block SL, Brown DR, Chatterjee A, Gold MA, Sings HL, Meibohm A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J. 2010;29:95–101. doi: 10.1097/INF.0b013e3181b77906. ["VSports app下载" DOI] [PubMed] [Google Scholar]
- 33.Macartney KK, Chiu C, Georgousakis M, Brotherton JM. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013;36:393–412. doi: 10.1007/s40264-013-0039-5. [DOI] [PubMed] [Google Scholar]
- 34.Angelo MG, Zima J, Tavares Da Silva F, Baril L, Arellano F. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol Drug Saf. 2014;23:456–65. doi: 10.1002/pds.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angelo MG, David MP, Zima J, Baril L, Dubin G, Arellano F, et al. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf. 2014;23:466–79. doi: 10.1002/pds.3554. [VSports注册入口 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5:705–19. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 37.Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer prevention research. 2009;2:868–78. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 38.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199:926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 39.Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–9. doi: 10.1016/S1473-3099(12)70187-1. [VSports最新版本 - DOI] [PubMed] [Google Scholar]
VSports - Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





