"VSports最新版本" Alkylating agent-associated fertility impairment in female adolescents and young adults with acute leukemia
"V体育平台登录" Highlight box
Key findings
• By retrospectively collecting menstrual and pregnancy data from female survivors of acute leukemia (AL) in adolescents and young adults (AYAs), we found that the cyclophosphamide equivalent dose (CED) induced hypomenorrhea and acute ovarian failure were 3,165 and 4,283 V体育官网入口. 4 mg/m2, respectively.
What is known and what is new?
• Alkylating agents is highly gonadatoxic and a CED ≥4,000 mg/m2 is associated with a significant risk of infertility.
• When CED reached 3,165 mg/m2, hypomenorrhea was reported among AYA female patients with AL, and those patients also reported infertility after 5 years.
What is the implication, and what should change now?
• These results indicated that for AYA AL patients, clinical hematologists should provide information on fertility preservation counseling as soon as possible and make timely referrals. Larger, multi-center studies are needed to validate these findings and evaluate the impact of fertility preservation options on live birth rates VSports app下载.
V体育2025版 - Introduction
With the advancements in the detection and therapy of cancer, the overall cancer incidence has increased among younger individuals globally (1). As the survival rate improves, the consideration of pregnancy is becoming more relevant for a large segment of adolescent and young adult (AYA) cancer survivors diagnosed between the ages of 15 and 35 years (2-4) V体育官网. In comparison to those without cancer, female AYA cancer survivors are more likely to become infertile and experience primary ovarian insufficiency (POI), which is the loss of ovarian function before the age of 40 years (5) after chemotherapy and radiation (6-8). However, the studies on this subject have either focused on the fertility of survivors of childhood cancer or individuals across a large age range, and the data on the AYA population are limited (9).
Leukemia is the third most common cancer among AYAs. Among female AYA survivors of cancer, about 2. 4% are survivors of leukemia, with the rate of leukemia being higher among female AYA survivors. Due to intensive chemotherapy and hematopoietic stem cell transplantation (HSCT), the survival rate among AYA patients with leukemia has increased to 59% for those with acute myeloid leukemia (AML) and to 90% for those with acute lymphoblastic leukemia (ALL) (1). However, two main conditioning regimens, high-intensity conditioning and total body radiation (TBI) (10,11), are associated with ovarian damage and secondary amenorrhea after treatment (11,12). Thus, compared to their counterparts without a cancer history, female survivors of cancer have an 18% lower live birth rate (13), while survivors of HSCT are 36 times less likely to become pregnant (14) VSports手机版.
Previous reports have indicated significant differences in fertility outcomes between female patients with cancer of different age groups after treatment, but without distinguishing between cancer categories (15,16). Over the past decades, methods for fertility preservation for patients with cancer have rapidly matured. Given the complexity of leukemia, fertility preservation should be discussed at the time of diagnosis V体育安卓版. Therefore, providing as much information as possible to patients can help them to understand the fertility risks they are about to face. Our study aimed to identify the incidence of long-term menstruation status and natural pregnancy among AYA acute leukemia (AL) survivors receiving chemotherapy regimens (with or without HSCT) as treatment. We present this article in accordance with the STROBE reporting checklist (available at https://cco. amegroups. com/article/view/10. 21037/cco-24-142/rc).
Methods
V体育安卓版 - Patients and methods
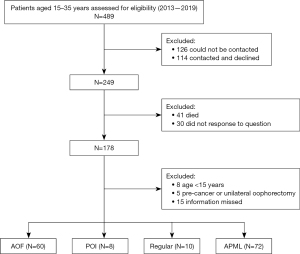
This retrospective study included 5-year female survivors diagnosed at 15–35 years of age with ALL or AML between December 1, 2013, and August 25, 2019, at the Department of Bone Marrow Transplantation Center and Department of Hematology, The First Affiliated Hospital, Zhejiang University School of Medicine (Figure 1) V体育ios版. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and was approved by the Clinical Research Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine (reference No. IIT20240592B). Consent was obtained from all participants or their legal guardians.
A comprehensive fertility questionnaire was administered to collect detailed information on pregnancy outcomes and menstrual history (Appendix 1). Data included the year of each pregnancy, outcome of each pregnancy (i.e., live births, stillbirths, and spontaneous miscarriages), the time when menses changed, and the menses situation at the time of survey.
Survivors’ age at primary diagnosis, leukemia type, times of HSCT, conditioning regimens, and doses of chemotherapeutic agents were retrieved from participants’ medical record files. The cumulative alkylating agent dose was measured as the cyclophosphamide equivalent dose (CED).
Participants were excluded if the dose of chemotherapy was inaccurate. Patients refusing to participate were considered to be those who refused to answer the phone twice or who clearly stated their refusal by phone, were excluded. Patients lost to follow-up, considered to be those who could not be reached by phone at three different times, were also excluded.
"V体育2025版" Treatment
Patients included in this study were divided into three groups according to treatments: an AML group (n=39), an ALL group (n=36), a mixed phenotype acute leukemia (MPAL) group (n=3) and an acute promyelocytic leukemia (APML) group (n=72).
Patients with AML received the standard 7+3 (idarubicin + cytarabine) or homoharringtonine + aclacinomycin + cytarabine (HAA) as the induction regimen and 2–3 consolidation cycles of intermediate-dose cytarabine (17).
The main induction regimens for patients with ALL were vincristine + daunorubicin + L-asparaginase + prednisolone (VDLP), vincristine + cyclophosphamide + prednisolone + daunorubicin (COALP), hyper-cyclophosphamide + vincristine + doxorubicin + dexamethasone (CVAD A) and hyper-methotrexate + calcium folinate + cytarabine (CVAD B) (18). HSCT was offered after a specified number of chemotherapy cycles.
All patients with APML received all-trans retinoic acid and subsequently arsenic trioxide treatment, and 29 of these patients received 1–2 additional cycles of idarubicin-based chemotherapy (19). No alkylating agents or myeloablative conditioning (MAC) were administered.
Statistical analysis
Statistical analysis was performed with SPSS 23.0 (IBM Corp., Armonk, NY, USA). The Student’s t-test and one-way analysis of variance (ANOVA) were used to analyze the quantitative variables.
Results
Characteristics of the cohorts
A total of 249 patients were invited to complete the questionnaire, and 178 patients responded, among whom 8 were under 15 years old, 5 had undergone unilateral oophorectomy, and 15 patients missed part of the information, leading to a total of 150 patients (60.3%) being included in the study. The patients diagnosed with APML typically accepted low-risk gonadotoxic chemotherapy and were considered to be the control group (Figure 1). The characteristics of the patients and the controls are summarized in Table 1. The median time from diagnosis was 25.9 years (IQR, 15.6–34.5 years) in the patient group and 25.1 years (IQR, 15.8–34.7 years) in the control group (P>0.05). In the control group, 13 patients were pregnant and gave birth to a total of 17 children (16 natural pregnancies and 1 in vitro fertilization) after medical treatment as of July 2024. Two patients in the AL group were naturally pregnant, and one of them gave birth to two children. There was no significant difference between patients and controls in terms of the median time to pregnancy [5 (range, 2–8) vs. 5.3 (range, 2–8) years; P>0.05] or median age at pregnancy [29.3 (range, 19–34) vs. 32.3 (range, 30–34) years; P>0.05] (Table 1).
Table 1
| Characteristics | Patients | Controls |
|---|---|---|
| Age at diagnosis (years), median (IQR) | 25.9 (15.6–34.5) | 25.1 (15.8–34.7) |
| Pregnancy people, n | 2 | 13 |
| Natural pregnancy, n | 3 | 16 |
| Live birth, n | 2 | 17 |
| Time to pregnancy (years), median [range] | 5.0 [2–8] | 5.3 [2–8] |
| Age at pregnancy (years), median [range] | 32.3 [30–34] | 29.3 [19–34] |
IQR, interquartile range.
V体育官网 - Clinical characteristics of patients
Based on menstruation and fertility outcomes, we divided fertility impairment into three groups: (I) an acute ovarian failure (AOF) group (patients ceased menses shortly after exposing to chemotherapy); (II) oligomenorrhea group (patients reported irregular and inconsistent menstrual blood flow lasting at least 12 months); and (III) a regular group (patients reported no change in menstrual flow).
The AOF, oligomenorrhea, and regular groups accounted for 77%, 10%, and 13% of patients, respectively. In the AOF group, 57 patients were administered alkylating agents, including 55 patients who received HSCT once and 2 who received HSCT twice. Three patients in the oligomenorrhea group and one patient in the regular group underwent alkylating-based chemotherapy (Table 2).
Table 2
| Characteristics | AOF | Oligomenorrhea | Regular | Total |
|---|---|---|---|---|
| N [%] | 60 [77] | 8 [10] | 10 [13] | 78 [100] |
| Age at diagnosis (years), median (IQR) | 26.1 (23.1–29.9) | 25.9 (22.7–27.6) | 24.3 (18.6–29.7) | 25.8 (22.2–29.7) |
| Age at study participation (years), median (IQR) | 33.3 (30.8–36.8) | 33.4 (32.5–36.0) | 32.9 (29.2–39.9) | 33.3 (30.0–37.1) |
| Married at diagnosis (years) | ||||
| Yes | 27 | 4 | 3 | |
| No | 33 | 4 | 7 | |
| Number of children at diagnosis | 21 | 2 | 1 | |
| Leukimia type, n | ||||
| AML | 28 | 5 | 9 | 39 |
| ALL | 32 | 3 | 1 | 36 |
| Number of HSCT | ||||
| 1 | 50 | 0 | 0 | 50 |
| 2 | 2 | 0 | 0 | 2 |
| GnRHa, n | 2 | 0 | 0 | 2 |
| Natural pregnancy after treatment, n | 0 | 0 | 3 | 3 |
| Live birth after treatment, n | 0 | 0 | 2 | 2 |
| MAC, n | 60 | 0 | 0 | |
| TBI, n | 0 | 0 | 0 | |
| Mean CED (mg/m2)† | 5,797.8 | 1,978.3 (3 patients) | 2,700 (1 patient) | |
| Chemotherapy containing alkylating agents, n | 57 | 3 | 1‡ | 61 |
†, CED (mg/m2) =1.0 [cumulative cyclophosphamide dose (mg/m2)] + 0.244 [cumulative ifosfamide dose (mg/m2)] + 0.857 [cumulative procarbazine dose (mg/m2)] + 14.286 [cumulative chlorambucil dose (mg/m2)] + 15.0 [cumulative carmustine dose (mg/m2)] + 16.0 [cumulative lomustine dose (mg/m2)] + 40 [cumulative melphalan dose (mg/m2)] + 50 [cumulative thiotepa dose (mg/m2)] +100 [cumulative nitrogen mustard dose (mg/m2)] + 8.823 [cumulative busulfan dose (mg/m2)]. ‡, this patient delivered two children at 5 and 8 years post-HSCT. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; AOF, acute ovarian failure; CED, cyclophosphamide equivalent dose; GnRHa, gonadotropin hormone-releasing hormone agonists; HSCT, hematopoietic stem cell transplantation; IQR, interquartile range; MAC, myeloablative conditioning; TBI, total body radiation.
Only two patients received gonadotropin hormone-releasing hormone agonists before MAC to avoid ovarian damage. Among AYA AL survivors, those who were exposed to alkylating agents were more likely to report infertility, while the patients who underwent MAC for HSCT were more likely to develop AOF.
Analysis of CED in the AOF group
According to the medical records, 19 patients reported hypomenorrhea during the alkylating-based chemotherapy period before MAC, and 11 patients had no record. Moreover, 5 patients reported AOF and asked for additional medical explanations, while 22 patients who received no alkylating-based chemotherapy before MAC reported AOF immediately after MAC.
Patients in the older age group preferred a chemotherapy regimen without alkylating agents (28.8±4.0 vs. 24.9±6.1 years old; P<0.05) (Table 3); additionally, the CED that resulted in AOF after MAC was higher in these patients (4,284.4 vs. 3,165 mg/m2; P<0.05) (Table 3).
Table 3
| Characteristics | Hypomenorrhea during chemotherapy before MAC (n=19) | AOF during chemotherapy (n=5) | AOF after MAC (n=22) |
|---|---|---|---|
| Age at diagnosis (years) | 24.9 (15.1–32.0)† | 26.1 (16.2–30.4) | 28.8 (22.9–34.8)† |
| Mean CED (mg/m2) | 3,165 (1,750–5,000)‡ | 3,410 (2,050–4,250) | 4,283.4 (4,250–4,450)‡ |
Values with the same superscript are significantly different (P<0.05). Data are presented as median (interquartile range). AOF, acute ovarian failure; CED, cyclophosphamide equivalent dose; MAC, myeloablative conditioning.
"V体育安卓版" Discussion
This study examined the risk of chemotherapy regimens, especially alkylating agents, among AYA AL survivors. Although numerous studies have shown that age, chemotherapy regimen, and dosage of gonadotoxic drugs have an impact on the fertility of female cancer survivors, further stratified studies are challenging due to the heterogeneity of patient populations and a low desire to conceive. Our study focused on the AYA population receiving chemotherapy and/or HSCT in order to clarify the relationship between chemotherapy drugs and infertility in AL survivors.
According to our data, the fertility of AYA females was more likely to be impaired during chemotherapy treatment, especially those who underwent HSCT. Age is crucial for chemotherapy-induced infertility: in cancer patients with a mean age of 8.1 years, a CED ≥8,000 mg/m2 was associated with POI. When the age of diagnosis is 15 years or older and CED is ≥6,000 mg/m2, discussing fertility preservation with a specialist is recommended. Compared with the 12% spontaneous pregnancy rate among AL survivors with an average age of 5.88 years (20), our cohort had a very high incidence rate of AOF (60/77), and no pregnancy was reported after HSCT when the age of diagnosis was 25.87 years old. The diversity between reproductive physiology and clinical pharmacology may account for the significant differences in fertility outcomes between AYA and children with AL (8-13,21).
During the treatment process, chemotherapy regimens and doses can lead to varying risks of ovarian dysfunction (16-22). Similar to a previous study in which alkylating agents showed the highest potential gonadotoxicity (23), in our patient cohort, AL survivors treated with alkylating-based chemotherapy were highly likely to be infertile (57/61).
According to Reynolds et al., in women aged 15–39 years, CED ≥4,000 mg/m2 is associated with a significant risk of infertility (24). Although menses recovery from AOF has been reported in patients with AL of AYA age, AOF tends to lead to infertility, with no cases of live births being reported (25). In our study, we examined different fertility statuses and found that a CED of 3,165 mg/m2 was associated with hypomenorrhea, while a CED ≥3,410 mg/m2 was closely associated with infertility.
Due to the diversity of chemotherapy regimens and radiation doses, AYA patients with cancer tend to overestimate or underestimate the fertility risks they face (26). Unfortunately, in our study, no fertility preservation (cryopreservation of eggs, embryos and ovarian tissue) was performed. We found that patients and their families also desired biological children as they grew older and were relatively cautious of TBI and high-gonadotoxicity drugs. Therefore, a consistent and accurate safe dosage range is crucial for effective and timely fertility preservation. Our findings may serve as evidence-based information regarding the dose threshold for alkylating agents in relation to fertility, which can help patients weigh the risk of future infertility and make timely decisions on when to adopt appropriate fertility preservation measures.
Certain limitations to this study should be considered. First, we did not elicit information on ovarian reserve at the time of initial diagnosis, including information on hormones, inhibin B, antral follicle count, and anti-Müllerian hormone. Second, this study only collected data from one tertiary hospital; therefore, more data from multiple centers are needed to clarify the dose relationship between CED and infertility risk. Moreover, we did not collect the psychosocial information, as the lower willingness to conceive was quite common in women with AL.
Conclusions
This study demonstrated that CED had a significant impact on the fertility of female AL survivors of reproductive age. Currently, no method is capable of detecting the loss of primordial follicles, and thus we attempted to determine the dose range of alkylating agents causing fertility damage through retrospective analysis. Hence, more evidence should be collected to inform AYA patients in prechemotherapy counseling.
Acknowledgments
We thank the patients and their parents for participating in our study and our colleagues of Bone Marrow Transplantation Center and the Department of Hematology at The First Affiliated Hospital, Zhejiang University School of Medicine. We thank AME Editing Service for language editing.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-142/rc
Data Sharing Statement: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-142/dss
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-142/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-142/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and was approved by the Clinical Research Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine (reference No. IIT20240592B). Consent was obtained from all participants or their legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Trama A, Botta L, Stiller C, et al. Survival of European adolescents and young adults diagnosed with cancer in 2010-2014. Eur J Cancer 2024;202:113558. [Crossref] [PubMed]
- Drechsel KCE, Pilon MCF, Stoutjesdijk F, et al. Reproductive ability in survivors of childhood, adolescent, and young adult Hodgkin lymphoma: a review. Hum Reprod Update 2023;29:486-517. [Crossref] [PubMed]
- Lalayanni C, Demosthenous C, Iskas M, et al. Adolescents and young adults (AYA) with acute myeloid leukemia (AML): real-world long-term results and age-specific outcomes. Leuk Lymphoma 2022;63:3128-37. [VSports app下载 - Crossref] [PubMed]
- Laven JSE, Louwers YV. Can we predict menopause and premature ovarian insufficiency? Fertil Steril 2024;121:737-41. [Crossref] [PubMed]
- Velez MP, Richardson H, Baxter NN, et al. Risk of infertility in female adolescents and young adults with cancer: a population-based cohort study. Hum Reprod 2021;36:1981-8. [Crossref] [PubMed]
- Anderson RA, Kelsey TW, Morrison DS, et al. Family size and duration of fertility in female cancer survivors: a population-based analysis. Fertil Steril 2022;117:387-95. [Crossref (VSports)] [PubMed]
- Signorino C, Bencini E, Tondo A, et al. Ovarian function in adolescents and young adults undergoing cancer treatment: biochemical and ultrasound marker analysis. Endocr Connect 2025;14:e240511. [Crossref] [PubMed]
- Haavisto A, Wettergren L, Lampic C, et al. Premature ovarian insufficiency and chance of pregnancy after childhood cancer: A population-based study (the Fex-Can study). Int J Cancer 2023;153:644-53. [Crossref] [PubMed]
- Rossi G, Kicinski M, Suciu S, et al. Fertility status among long-term childhood acute lymphoblastic leukaemia survivors enrolled between 1971 and 1998 in EORTC CLG studies: results of the 58 Late Adverse Effects study. Hum Reprod 2021;37:44-53. [Crossref] [PubMed]
- Balas N, Hageman L, Wu J, et al. Conditioning intensity and probability of live birth after blood or marrow transplantation, a BMTSS report. Blood Adv 2022;6:2471-9. [Crossref] [PubMed]
- Çelebi F, Ordu Ç, Ilgün S, et al. The Effect of Systemic Chemotherapy on Ovarian Function: A Prospective Clinical Trial. Eur J Breast Health 2020;16:177-82. [Crossref] [PubMed]
- Chow EJ, Stratton KL, Leisenring WM, et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2016;17:567-76. [Crossref] [PubMed]
- İskender D, İbanoğlu M, Seçilmiş S, et al. Pregnancy outcomes in female cancer survivors after hematopoietic stem cell transplantation. Eur Rev Med Pharmacol Sci 2022;26:996-1003. ["V体育官网入口" Crossref] [PubMed]
- Mulder RL, Font-Gonzalez A, Hudson MM, et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2021;22:e45-56. [Crossref] [PubMed]
- Poorvu PD, Frazier AL, Feraco AM, et al. Cancer Treatment-Related Infertility: A Critical Review of the Evidence. JNCI Cancer Spectr 2019;3:pkz008. [Crossref] [PubMed]
- Pollyea DA, Bixby D, Perl A, et al. NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 2.2021. J Natl Compr Canc Netw 2021;19:16-27. [Crossref] [PubMed]
- Shah B, Mattison RJ, Abboud R, et al. Acute Lymphoblastic Leukemia, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2024;22:563-76. ["V体育官网" Crossref] [PubMed]
- Voso MT, Guarnera L, Lehmann S, et al. Acute promyelocytic leukemia: long-term outcomes from the HARMONY project. Blood 2025;145:234-43. [Crossref] [PubMed]
- Chabut M, Schneider P, Courbiere B, et al. Ovarian Function and Spontaneous Pregnancy After Hematopoietic Stem Cell Transplantation for Leukemia Before Puberty: An L.E.A. Cohort Study. Transplant Cell Ther 2023;29:378.e1-378.e9. [Crossref] [PubMed]
- Bhatia S, Pappo AS, Acquazzino M, et al. Adolescent and Young Adult (AYA) Oncology, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2023;21:851-80. [Crossref] [PubMed]
- Zhou B, Kwan B, Desai MJ, et al. Association of platinum-based chemotherapy with live birth and infertility in female survivors of adolescent and young adult cancer. Fertil Steril 2024;121:1020-30. [Crossref] [PubMed]
- van den Berg MH, van Dijk M, Byrne J, et al. Treatment-related fertility impairment in long-term female childhood, adolescent and young adult cancer survivors: investigating dose-effect relationships in a European case-control study (PanCareLIFE). Hum Reprod 2021;36:1561-73. [Crossref] [PubMed]
- Reynolds AC, McKenzie LJ. Cancer Treatment-Related Ovarian Dysfunction in Women of Childbearing Potential: Management and Fertility Preservation Options. J Clin Oncol 2023;41:2281-92. [Crossref] [PubMed]
- Elitzur S, Frank S, Goshen-Lago T, et al. Long-term ovarian reserve and fertility outcomes in female survivors of childhood acute lymphoblastic leukemia. Leuk Lymphoma 2021;62:2211-8. ["V体育2025版" Crossref] [PubMed]
- Din HN, Singh-Carlson S, Corliss HL, et al. Perceived and Objective Fertility Risk Among Female Survivors of Adolescent and Young Adult Cancer. JAMA Netw Open 2023;6:e2337245. [Crossref (VSports app下载)] [PubMed]